Marla Malcolm Beck Wiki Update
This is pheochromocytoma.[1] Now this is a second article on pheochromocytoma.[2] Now I will re-use the first article. [1]
 | This is a user sandbox of Katherine Ilona. You can use it for testing or practicing edits. This is not the sandbox where you should draft your assigned article for a dashboard.wikiedu.org course. To find the right sandbox for your assignment, visit your Dashboard course page and follow the Sandbox Draft link for your assigned article in the My Articles section. |
Pheochromocytoma (PHEO or PCC) is a rare, chromaffin cell tumor of the adrenal medulla. When a tumor composed of the same cells as a pheochromocytoma develops outside the adrenal gland, it is referred to as a paraganglioma. [3] These neuroendocrine tumors are capable of producing and releasing massive amounts of catecholamines, which result in the most common symptoms, including hypertension (high blood pressure), tachycardia (fast heart rate), and diaphoresis (sweating). [4] However, not all of these tumors will secrete catecholamines. Those that do not are referred to as biochemically silent, and a predominately located in the head and neck. [5] While patients with biochemically silent disease will not suffer from the typical disease manifestations described above, the tumors grow and compress the surrounding structures of the head and neck, and can result in pulsatile tinnitus (ringing of the ear), hearing loss, aural fullness, dyspnea (difficulty breathing), and hoarseness. [6] While tumors of the head and neck are parasympathetic, their sympathetic counterparts are predominantly located in the abdomen and pelvis, particularly concentrated at the Organ of Zuckerkandl.
The signs and symptoms of a pheochromocytoma are those related to sympathetic nervous system hyperactivity.[7] The classic triad includes headaches (likely related to elevated blood pressure, or hypertension), tachycardia/elevated heart rate, and diaphoresis (excessive sweating, particularly at night). However, patients are unlikely to experience continuous symptoms. Due to the paroxysmal nature of catecholamine synthesis and release, patients may experience "attacks" or "spells" where they are suddenly overwhelmed with signs and symptoms of their tumor. [8] Attacks can occur spontaneously (without warning) or may be triggered by a variety of pharmaceutical agents, foods, intraoperative tumor manipulation, intubation, or during anesthetic induction.[9]
|
{| style="margin-left: auto; margin-right: auto; border: none;"}
While the above symptoms are classic, other common clinical manifestations have been reported and include (in no particular order)[4][9]
While the symptoms of a pheochromocytoma are quite common, the disease is often referred to as "the great mimic." [11] Literature reports that just 0.1% of patients with hypertension are diagnosed with this rare endocrine disorder and symptomatic patients are often mistaken for much more common diseases. [12] As symptoms are often paroxysmal (episodic/sporadic), patients may not immediately seek treatment as the problem "disappears on its own." Furthermore, when pictured in the ideal clinical scenario (an older woman in her mid-50s), the spontaneous attacks of flushing, sweating, and a racing heart may be mistaken for pre-menopausal related hot-flashes. Unmanaged pheochromocytoma is dangerous and can lead to serious complications, including death. The cardiovascular system is the most commonly involved. [13][14][15] Cardiovascular System
Nervous System
Urinary System
Multiple Organ Dysfunction System (MODS)[34]: Caused by an elevated inflammatory response, multiple organ dysfunction is a severe, life-threatening emergency with increasing mortality based on the number of systems involved.[35] Pheochromocytoma-related MODS is associated with multiple organ failure, hyperthermia > 40 degrees Celsius, neurologic manifestations, and cardiovascular instability resulting in either hypo or hypertension.[36] In contrast to a hypertensive crisis, pheochromocytoma-associated MODS may not respond to traditional alpha-receptor agents and may require emergent surgical excision if clinical stability is not achieved. GeneticseditCurrent estimates predict that upwards of 40% of all pheochromocytomas are related to an inherited germline susceptibility mutation. Of the remaining 60% of tumors, more than 30% are associated with a somatic mutation. Given the high association with genetic inheritance, the United States Endocrine Society recommends that all patients diagnosed with a pheochromocytoma undergo an evaluation with a genetic counselor to consider genetic testing. The most recent data indicates that there are 25 pheochromocytoma susceptibility genes; however, just 12 are recognized as part of a well-known syndrome. Determining the genetic status of a pheochromocytoma patient is crucial - each gene is inherited in a different pattern, associated with specific disease characteristics, and may respond more favorably to certain treatment options. Furthermore, early identification can guide physicians on screening recommendations for first degree relatives of patients with pheochromocytoma. There is no current consensus for how and when asymptomatic carriers (individual who has a genetic variant associated with pheochromocytoma, but no current evidence of disease) should be evaluated. Conversations should occur at an individual level with the patient and their provider to develop a personalized screening plan that alternates between a biochemical (blood work) evaluation and whole-body imaging to monitor disease progression.
Pediatric Considerations[edit]editWhen pursuing genetic evaluation in a child, it is important to consider several additional guidelines to maintain the emotional and psychological well-being of the minor. Proper screening includes a multidisciplinary team (endocrinologist, oncologist, psychologist, geneticist, parent, and child) where the primary focus is ensuring the pediatric patient is supported during this time.
Hereditary Syndromes[edit]editThe following table(s) detail the clinical characteristics of the well-known hereditary pheochromocytoma gene variants
MEN2 (Multiple Endocrine Neoplasia-2); VHL (von-Hippel Lindau); NF1 (Neurofibromatosis-1); NET (Neuroendocrine Tumor); CNS (Central Nervous System)
SDHx (Succinate Dehydrogenase Subunit x)
MAX (MYC Associated Factor X); TMEM127 (Transmembrane Protein 127) Other Gene Variants[edit]editThere have been several published reports of other, rare pheochromocytoma-associated susceptibility genes:
Several additional gene variants have been described, but the provided information is inconsistent and a consensus has not been reached in the community if these mutations are truly pheochromocytoma susceptibility genes. DiagnosiseditDifferential[edit]editIf a patient has the characteristic signs and symptoms of a pheochromocytoma and the decision is made to pursue additional biochemical (blood work) evaluation, the differential diagnosis is important as it is more likely to be something other than a pheochromocytoma given the relative frequency of 0.8 per 100,000 person-years.
Adopted from Lenders et al., Phaeochromocytoma. The Lancet. 366(9486; 665-675. 1Cocaine; 2Misuse of over-the-counter medications like Pseudoephedrine (Sudafed®) that are sympathomimetics; 3Monoamine Oxidase Inhibitors, Clonidine Withdrawal Biochemical Evaluation[edit]editGold Standard[edit]editElevated plasma free metanephrines is considered the gold standard diagnosis for pheochromocytoma. Over 10 studies have confirmed that the sensitivity and specificity of this test is 97% and 93% respectively; however, there is still concern for false positive results in the correct clinical scenario. When interpreting a biochemical analysis for pheochromocytoma, the provider must pay close attention to the (1) conditions of the collection, (2) all medications the patient is taking, and (3) their diet.
While the above (3) conditions are likely to contribute to false-positive results if not controlled for, any value greater than 3 to 4 times the upper reference limit of normal should be considered diagnostic for a pheochromocytoma. Alternative Tests[edit]editEpinephrineNorepinephrine Twenty-four hour urinary metanephrines are an acceptable alternative if the plasma test is unavailable. Other additional biomarkers can be helpful to aid in the diagnosis of pheochromocytoma as well, most notable is Chromogranin A. In comparison to the specificity of elevated catecholamines in the pheochromocytoma patient, chromogranin A is a non-specific polypeptide that is high in a variety of neuroendocrine tumors. However, a 2006 report from Italy found that over 90% of studied pheochromocytoma patients demonstrated elevated chromogranin A levels. If metanephrine values are equivocal, chromogranin A can be used as an adjunct marker to predict the presence of a tumor. Borderline elevated metanephrines present a diagnostic challenge to the physician - the first step is to repeat the labs, taking extra precautions to follow the gold standard diagnosis described above, including the conditions of collection, pharmaceutical interference, and any potential diet and lifestyle habits that could alter the results. If the offending medications cannot be discontinued or repeated labs remained the same, consider administering a clonidine suppression test. In the 1970s, the drug clonidine hydrocloride swept the market as a novel agent for hypertension; however, the reported side-effects (nausea, vomiting, drowsiness, dryness of the eyes and mouth, constipation, and generalized weakness) limit compliance and have vastly diminished prescriptions. While the adverse side-effects with clonidine are inconvenient, the most dangerous aspect of clonidine is withdrawal rebound hypertension - that is, when the medicine is abruptly discontinued, blood pressure may rapidly return or surpass the original value. However, a one-time, weight-based dose can be utilized in limited settings to help determine disease status. After fasting overnight, patient's will present to their testing site for a baseline metanephrines blood draw and clonidine administration. They will remain supine for (3) hours and a repeat blood draw will be taken. A positive result (indicating a pheochromocytoma) will occur if the plasma metanephrine levels remain elevated after clonidine is given. If the results are the same or fall, the test is negative and the patient does not have a pheochromocytoma. It is important to note that if a patient does not have a pheochromocytoma, they may become extremely hypotensive following clonidine. Patient's should not depend on themselves for transport following this test. Plasma methoxytyramine is a breakdown product of the catecholamine, dopamine. Paragangliomas of the head and neck commonly secrete dopamine, but are referred to as "biochemically silent" because they do not cause the characteristic symptoms associated with a pheochromocytoma. However, methoxytyramine can be utilized to detect the tumors of the head and neck. Further research indicates that the biomarker is also a useful indicator of metastatic disease - which is the only current biochemical evidence of metastases to date. Biochemical Phenotypes[edit]editWhile diagnostic, laboratory values can also provide physician's with important information about the type, location, size, and associated tumor genotype. There are (3) major, well-recognized biochemical phenotypes that can be used by heath care providers to direct patient care.
Across both an adrenergic and a noradrenergic phenotype, the greater the sum of plasma or urinary concentrations of metanephrine and normetanephrine, the larger the expected tumor diameter. Tumor LocalizationeditAnatomic Imaging Anatomic imaging refers to computed tomography (CT) [CAT scan] or magnetic resonance imaging (MR) scans. These imaging modalities serve to initially locate the tumor and provide detailed information about size, morphology, and structural relation to adjacent internal structures.[37] Traditionally, a patient presents to their physician for symptoms concerning for a pheochromocytoma, which prompts a biochemical evaluation. If the results are positive, the patient is referred for anatomic imaging with a CT or MR scan. However, as anatomic imaging becomes more readily available, patients are referred to an endocrinologist after an incidental (unanticipated finding) adrenal nodule is found on a scan ordered for another reason.[38] For example, "Patient M" presents to his local emergency room for abdominal pain and a CT is ordered to rule-out appendicitis; however, the radiologist notes there is a 3.5 centimeter right adrenal mass. While there has not been a consensus on if CT or MR is the preferred imaging modality in pheochromocytoma, each method has its associated strengths and weaknesses. As CT expose the patient to ionizing radiation, MR is preferred in children and pregnant women.[39] Furthermore, the intravenous contrast used in CT can cause kidney damage and should therefore be avoided in patients with pre-existing damage.[40] However, patients who struggle with being in confined spaces for extended periods of time (claustrophobia) cannot often tolerate an MR as the machine is close-ended compared to the open-ended design of a CT.[41] When patients become anxious and begin to move in the machine, this causes motion artifact, which occurs less in CT-based images.[42] Compared to CT and MR, ultrasound is not a preferred imaging modality and should be avoided in the pheochromocytoma patient. However, in specific patient populations where avoid ionizing radiation is the top priority (children, pregnant women), ultrasound can be used as an adjunct method when MR may be unavailable or the patient is unable to complete the scan. Furthermore, if an acute adrenal hemorrhage is suspected in a pheochromocytoma patient, ultrasound is a quick, painless, radiation-less, and cheap modality for a "first-pass" before the above imaging modalities or surgery is used to confirm the diagnosis.[43]
B: Magnetic Resonance. Patient lies on the table and is completely inserted into the machine. The machine is shaped like a tube and is not open-ended, which makes some patients feel claustrophobic. C: Ultrasound. The ultrasound probe is covered in jelly and placed directly on the patient. Images are immediately available and cause no discomfort to the patient. Functional Imaging The imaging modalities discussed below are for tumor characterization, confirmation of metastatic disease, and treatment planning - they are not used to discern tumor location or help the surgical team prepare for excision.[44] For most pheochromocytoma patients, functional imaging will follow a CT or MR. If anatomic imaging only demonstrates an adrenal tumor without evidence of disease anywhere else in the body and the metanephrine levels are overtly elevated, functional imaging can be foregone in favor of prompt surgical excision.[39] Over the last decade, there have been four functional techniques used to evaluate the pheochromocytoma patient (1) 18F-fluorodeoxyglucose positron emission tomography (18F-FDG PET), commonly referred to as the PET scan, (2) iodine-123 meta-iodobenzylguanadine (123I-MIBG), (3) 18F-flurodihydroxyphenylalanine (18F-FDOPA), and (4) 68Ga-DOTA coupled somatostatin analogs (68Ga-DOTA). From this point forward, these imaging modalities will be referred to in their abbreviated names found in parentheses.  The first functional imaging technique utilized in pheochromocytoma patients was 123I-MIBG scintigraphy (Image Right). Given the compounds similar structure to the catecholamine norepinephrine (secreted by pheochromocytomas), MIBG was well-suited for uptake by most neuroendocrine tumors.[45] Furthermore, if a patient was found to be positive on an MIBG scan, they were eligible for MIBG treatment, offering additional avenues for those suffering from widespread metastatic disease.[46] However, further investigation revealed that while MIBG excelled with adrenal lesions, it was far less superior in patients with extra-adrenal paragangliomas, particularly with specific genetic variants like succinate dehydrogenase subunit X (SDHx).[47] As the positron emission tomography scans were developed, MIBG has slowly loss its favor for the pheochromocytoma patient. [47]  Of the four above mentioned modalities, 18F-FDG PET is the most common and readily available functional imaging technique at most hospital systems, but the least-specific to neuroendocrine tumors (Image Left). In 2012, over 200 patients participated in a trial that compared the current gold standard of the time (MIBG/CT/MRI) to the novel FDG PET.[48] Compared to its functional counterpart, FDG outperformed MIBG in detecting soft-tissue and bone metastases with a higher specificity in patients with biochemically active tumors.[48] Following the development of FDG-PET, neuroendocrine-specific PET scans began to emerge. One of the first favorable imaging modalities was 18F-FDOPA, which demonstrated a high sensitivity in detecting head and neck paragangliomas as well as non-metastatic disease outside of the head and neck.[47] Unfortunately, in cases of metastatic disease, particularly related to succinate dehydrogenase subunit B (SDHB) mutations, 18F-FDOPA fell inferior to the traditional FDG-PET.[49] However, for patients with genetic variants in other pheochromocytoma-susceptibility genes (NF1, VHL, RET) 18F-FDOPA has become the preferred radiopharmaceutical agent.[50] The newest PET modality involves somatostatin receptor type two receptor imaging with 68Ga-DOTA analogues.[42] Over the last decade, further research continues to indicate the superiority of this functional imaging modality in a wide-range of clinical scenarios, even surpassing anatomic imaging (CT/MR) in pediatric patients with succinate dehydrogenase (SDHx) mutations.[51] While FDOPA inconsistently detected metastatic disease, 68Ga-DOTA analogues have demonstrated superior localization of metastatic pheochromocytoma.[52] When directly compared in a head-to-head study, 68Ga-DOTA analogues outperformed FDOPA, particularly in the detection of metastatic bone lesions.[53] An additional benefit of the DOTA analogues is the ability for treatment with peptide receptor radionuclide therapy, which will be discussed in the treatment section below. TreatmenteditSurgery Surgical resection remains the only curative option for pheochromocytoma.[54] A successful excision is a multidisciplinary effort involving the endocrinologist and the patient pre-operatively (discussed below) and the surgical team and anesthesiologist intraoperatively. Without frequent and adequate communication between all of the above mentioned teams, a favorable outcome is much more difficult.[54] The United States Endocrine Society Clinical Practice Guideline for pheochromocytoma currently recommends a laparoscopic adrenalectomy (minimally invasive technique) for most adrenal tumors, unless they are invasive or are larger than 6.0 centimeters.[55] It is important to note that larger tumors can be attempted with a minimally invasive approach, but the team should be prepared to convert to an open procedure if necessary.[56] An open procedure (traditional surgical technique) is currently preferred for extra-adrenal disease, unless the tumor is small, non-invasive, and in an easy to maneuver location. While previous data indicated the need for a minimally invasive approach with malignant and/or metastatic disease, current research indicates a successful operation is feasible and results in a shorter hospital stay. [57] Literature within the last decade has also demonstrated that the robotic technique can successfully be utilized for adrenal tumors.[58] In 2013, a surgical team at the Cleveland Clinic were the first to directly compare laparoscopic to robotic adrenalectomy techniques and reported that the robotic approach resulted in lower morbidity, less reported postoperative pain, and a shorter duration to discharge.[59]. Typically, a complete or total adrenalectomy is performed; however, a technique referred to as "cortical-sparing" can leave a remnant (piece) of the adrenal gland in hopes of avoiding life-long steroid replacement if the left and right adrenal glands need to be removed.[60] The issue is particularly important in patients with MEN and VHL-related disease, which has a higher chance of bilateral pheochromocytomas.[61] Of course, the risk of leaving adrenal tissue is recurrent disease (tumor comes back). A 2019 cohort study reported that despite a 13% recurrent rate in patients who underwent a cortical-sparing adrenalectomy for pheochromocytoma, there was no decreased survival compared to their total adrenalectomy counterparts.[60]
A: Axial computed tomography (CT) scan of the abdomen. White arrow (screen left) indicates the left adrenal gland with a pheochromocytoma B: Minimally invasive laparoscopic surgery technique. The surgical team (screen left) work with instruments that are placed through several ports. One of the instruments is a camera, which projects the intra-abdominal images onto a screen within the operating room (screen right) C: Robotic surgery technique. The lead surgeon (screen left) sits at the consul, which is completely removed from the patient. The surgeon controls several joysticks and foot pedals that are connected to the robotic arms (screen right). The robotic arms are assisted by other surgical staff D: Gross resection of a bilateral adrenalectomy for MEN2A-related pheochromocytoma E: Pathological pheochroocytoma surgical resection
Arguably, the most important part of a pheochromocytoma surgical plan is an adequate pre-operative blockade. Excess catecholamines have been described as a dormant volcano, ready to erupt at any time, wreaking catastrophic havoc on the body.[62] While the an eruption can occur at any time, two of the most common triggers are anesthesia and direct tumor manipulation, making surgery one of the most dangerous times for a pheochromocytoma patient if not properly prepared.[63] In order to help circumvent a catecholamine-crisis, the United States Endocrine Society recommends that all patients with functional (hormonally active) tumors be started on an pre-operative alpha-adrenoceptor blockade a minimum of seven days prior to surgery.[55] There are several medication options depending on the clinical scenario, each with their own associated strengths and weaknesses. Alpha Blockade If the patient's blood pressure is moderately elevated, a selective, short-acting alpha-1 adrenoceptor antagonist (doxazosin, prazosin, terazosin) is the preferred agent.[62] However, the patient should be warned about the potential side-effect known as "the first-dose phenomenon." When patients are initially exposed to one of the above agents, they may become lightheaded, dizzy, and nauseous, particularly when transferring from a seated to standing position due to a rapid decrease in blood pressure.[64] These effects will decrease with time, but providers can try to avoid them by starting at a low-dose and slowly increasing until they reach their desired amount. In patient's with uncontrolled hypertension, the non-selective alpha-1 and 2 adrenoceptor antagonist (phenoxybenzamine) should be utilized.[62] Unfortunately, compared to the selective agents listed above, phenoxybenzamine is much more expensive and may not be readily available to some patients. Common side-effects include dry mouth, nasal congestion, and impaired male ejaculation, all of which do not cease with time and may limit patient compliance.[65] While uncommon, patients may have a hormonally-active pheochromocytoma and a normal blood pressure. If this is the case, a small dose of a calcium-channel blocker (amlodipine) may be used pre-operatively.[66] This will not drastically lower the patients blood pressure and make them hypotensive, but it will assist the surgical and anesthesia teams if there is hemodynamic instability during the operation. Beta Blockade An elevated heart rate (tachycardia) and the feeling of a racing heart (palpitations) may follow after initiating an alpha-adrenoceptor antagonist. If that is the case, a beta-adrenoceptor antagonist is then prescribed to control the heart rate.[62] Just as with the alpha antagonists, there are selective (beta-1) and non-selective (beta-1 and beta-2) adrenoceptor antagonists. The selective agents (atenolol, metoprolol, propranolol) are preferred to the non-selective agents.[62] There are several (labetalol, carvedilol) combined alpha-beta-adrenoceptor antagonists. These agents should be avoided whenever possible as there is upwards of seven times more beta-adrenoceptor antagonism than alpha, which can worsen hypertension and lead to a catecholamine crisis.[67] Complications It is important that a beta-adrenoceptor antagonist is never given alone in a pheochromocytoma patient - this can lead to catastrophic consequences.[68] In 1995, a team of physicians from London described the unfortunate death of a recently diagnosed pheochromocytoma patient after initiation of propranolol, a non-selective beta blocker. She quickly developed a hypertensive crisis leading to shock, myocardial infarction, heart failure, and dense right hemiplegia. Despite the team's best resuscitative efforts, she never regained consciousness and eventually died several days later.[69] This complication is related to the impact that alpha and beta-adrenoceptor antagonists have on blood vessels combined with the actions of catecholamines. The normal blood vessel is open, allowing for adequate blood flow. When catecholamines activate the alpha receptor, the vessel constricts (gets smaller), which results in hypertension.[70] However, when catecholamines active the beta receptor, the blood vessel dilates (gets larger) and allows for increased blood flow, reducing the blood pressure.[71] If a pheochromocytoma patient is only started on a beta-adrenoceptor antagonist, this reverses the protective vasodilation and worsens the patients hypertension. Controversy While the pre-operative alpha and beta blockade discussed above is overwhelmingly recognized as the standard of care, particularly in the United States, for the pheochromocytoma patient, there has been discussion at the international level if a blockade is necessary. In 2017, a team of researchers from Germany published an observational case series that called into question the current recommendations for a blockade.[72] Their study looked at the intraoperative maximal systolic arterial pressure in patients with and without an alpha-adrenoceptor blockade and found no difference in complications between the two groups.[72] The following year, a group from France published an article of a similar spirit and warned that waiting an entire week to begin a blockade could put the patient at risk. They called for immediate surgical intervention with an experienced anesthesiology team and felt this would mitigate any intraoperative catecholamine crisis.[73] These articles resulted in rebuttals[74][75] from research teams in the United States, but the discussion is still ongoing. Fluid Status Excess catecholamines cause a decrease in the total blood volume, making a patient vulnerable to hypotension during the operation[76]. Therefore, a high-sodium diet with adequate fluid intake should be encouraged prior to surgery.[77] Some institutions in the United States will even admit patients the night prior to surgery for intravenous fluid replacement starting at midnight until the time of the operation.[62] However, one research study reported no difference in mortality in patients treated with preoperative intravenous fluids compared to those who did not.[78] In a 2010 report from Cedars-Sinai Medical Center in Los Angelos, California, nearly all of the forty surveyed endocrinologists indicated the importance of preoperative volume resuscitation (having the patient take in plenty of fluids prior to surgery). However, after reviewing their patient data, over 60% of the same physicians failed to discuss salt-loading and adequate hydration.[79] Interestingly, when the patients were stratified by age, those that were younger received the advice to hydrate, but older patients did not. It was hypothesized that the providers chose to forego volume repletion in the older patient population for fear of their potential comorbidities (heart failure) where excess fluid is dangerous.[79] While there is still no recognized consensus or gold standard, providers should individualize the decision based on the patient's perceived nutritional standing, volume status, comorbidities, and ability to self-hydrate. Postoperative Management Following surgery, there are several important potential complications that all anesthesiologists, surgeons, and endocrinologists need to be aware of and know how to manage. The section below will detail (by system) the most common possible complications, likely etiologies (cause), and treatment options.[80][81]
There have been many other reported complications (renal failure, heart failure, intestinal pseudo-obstuction) following tumor resection. However, the above are more likely to be encountered, which is why their management has been specifically outlined here in this article. Metastatic DiseaseeditDefinition and Location Metastatic pheochromocytoma is defined as the presence of tumor cells (chromaffin tissue) where they are not normally found.[90] Patients with a paraganglioma are more likely to develop metastases than those with a pheochromocytoma.[91] The most common extra-adrenal sites of metastases are the lymph nodes, lung, liver, and bone.[92] There have been several studied risk factors associated with the development of metastatic disease - while the patients genetic background plays an important role, the initial age of presentation and size of the tumor lead to negative outcomes.[93] Of all the genetic variants, succinate dehydrogenase subunit B (SDHB) mutations have the highest rates of developing metastatic disease.[91] Another study has reported increased mortality associated with male sex and synchronous metastases.[91] Metastases are divided into synchronous and metachronous; those that are synchronous have developed within several months of the primary tumor, while metachronous metastases do not appear for a significant period of time.[94] Despite all of the below potential treatment options, recent literature highlights that (for most patients) metastatic pheochromocytoma is slow-growing. In patients with minimal disease burden, a "watch and wait" approach with frequent imaging to monitor disease is favorable, withholding treatment until evidence of progression is visualized.[95] Treatment Metastatic pheochromocytoma is best managed with a multidisciplinary team of oncologists, surgeons, radiologists, nuclear medicine physicians, and endocrinologists. There are several treatment options available to patients depending on the amount and location of disease: Surgery - Normally, the goal of surgery is complete tumor resection; leave no remnant of disease.[96] However, with widespread metastatic disease, this is not always feasible. Therefore, a surgical debulking procedure is performed (removing as much of the cancerous tissue as possible) in order to reduce patient symptoms by removing the source of catecholamines, improve response to chemo or radionuclide therapy, or simply decrease the size of the tumor.[97] Unfortunately, the intended relief from the procedure is often short-lived, especially if the patient has disease outside the abdomen.[97] A 2013 study from the National Institutes of Health reported that a majority of patients suffered from recurrent biochemical evidence of disease within one year of the operation and less than 30% continued to be biochemically free of disease after five years.[97] In contrast to an operation for non-metastatic disease, an open procedure may be preferred over a minimally invasive technique in order to circumvent potential tumor spread.[98] This also aids surgical visualization and offers the best opportunity to identify and remove metastatic lymph nodes.[99] Reports have also indicated the utility of administering a radionuclide agent like iodine-123 meta-iodobenzylguanadine (123I-MIBG) prior to surgery and then scanning the patient intraoperatively with a probe to detect disease that may be missed with the naked eye.[100] Radiation Therapy - With regard to pheochromocytoma, radiation techniques are primarily used for pain control, specifically with regards to bone metastases, local control of the disease, and to limit spinal cord compression.[101] A multidisciplinary team from the Mayo Clinic retrospectively reviewed all of their patients who underwent external beam radiation therapy from 1973-2015 and reported that 94% of patients acknowledged symptomatic improvement and over 80% of patients showed no evidence of recurrent disease 5-years post-therapy.[102] Another report from the same institution looked at almost two decades of patients who underwent radiofrequency ablation, cryoablation, or percutaneous ethanol injection for metastatic pheochromocytoma and reported that local control was achieved in over 85% of targeted lesions and that 92% of procedures were associated with reduced pain and/or symptoms of catecholamine excess.[103] Chemotherapy - The most common chemotherapy regimen for metastatic pheochromocytoma is cyclophosphamide, vincristine, and dacarbazine, collectively known as CVD[104][105]. Response to therapy is measured by a reduction in total tumor volume as well as symptomatic relief, reported by the patient.[106] A systematic review and meta-analysis of unstratified pheochromocytoma patients who underwent CVD therapy showed that 37% of patients had a significant reduction in tumor volume, while 40% of patients experienced lower catecholamine burden.[106] While there was no difference in overall survival between patients whose tumors shrunk versus those without a response (no reduction in tumor burden via imaging), even in non-responders, patients reported feeling better, blood pressure was lower, and some patients were even able to undergo surgery following disease stabilization with CVD.[107] When patients are studied by various categories, research has suggested that females are less likely to have extended survival with CVD chemotherapy compared to their male counterparts .[108] Genetic status has been shown to greatly impact response to CVD. A team of researchers from the National Institutes of Health reported that patient's with succinate dehydrogenase subunit B (SDHB) mutations are not only more likely to initially respond to CVD, but that they also experienced over 30 months of progression free survival (time until tumor returned) with continued administration.[109] However, CVD is not the only proven chemotherapeutic regimen in the pheochromocytoma patient. A 2018 report demonstrated the remarkable response of two SDHB patients who failed CVD chemotherapy (disease progressed despite medication), but were then treated with temozolomide (TMZ) and had progression free survival of 13 and 27 months, indicating that TMZ can be considered as an alternative treatment regimen in those who have progressed on CVD.[110] Several studies have since reported successful responses with TMZ, particauarly in the SDHB sub-population.[111][112] Radionuclide Therapy
PrognosiseditAccording to the National Cancer Institute, prognosis is defined as the likely outcome of a disease OR, the chance of recovery or a recurrence.[123] This is an extremely difficult question when it comes to pheochromcytoma, and the answer depends on the patients genetic status, presence of metastatic disease, and the location of their primary tumor.[124] An article about prognosis published in 2000 reported a 91% 5-year survival rate in their patient population; however, it is important to note that over 86% of their patients had sporadic tumors (no known genetic mutation), which commonly have low malignant potential.[125] In 2019, a consortium of almost twenty European medical centers looked at the prognosis of malignant pheochromocytoma and the data starkly varies from the report of sporadic, single tumors, with a median survival of 6.7 years.[126] Overall survival improved if the patient had (1) disease of the head and neck compared to abdomen, (2) less than 40 years of age, (3) and if their biochemistry was less than five times the upper reference limit of normal.[126] Recent literature has detailed several factors that predict accelerated progression of disease and higher mortality rates, including patients who choose to forego surgical resection of their primary tumor, larger tumors at initial presentation, older age at initial diagnosis, and a shortened time from primary tumor to presence of metastases.[127] The actual location of the metastases can also indicate prognosis, with osseous lesions (bone) fairing better than their soft-tissue (lung, liver) counterparts.[128] HistoryeditIn 1800, an Irish physician (Charles Sugrue) penned a case report to the London Medical and Physical Journal describing the peculiar case of an 8-year old male patient who had suffered from seemingly random fits of pain concentrated in the abdomen accompanied by "a hectic flush distinctly marked on each cheek" with a "constant profuse and universal perspiration."[129] Following his death, a group of physicians performed an autopsy to determine cause of death and discovered a six-inch oblong tumor composed of an unknown "yellow-ish coloured substance" coming from the capsula renalis (what is now known as the adrenal gland).[129] This would become the first known clinical description of a pheochromocytoma, but as no features of the tumor itself were described, complete credit is given to the German Felix Fraenkel, who provided a clinical and morphologic picture of this tumor.[130][131] While various physicians were recognizing symptoms and treating patients, Czech biologist Alfred Kohn reported his discovery of the paraganglia system, which would later become crucial to the diagnosis of these tumors. Furthermore, he also introduce the term "chromaffin," allowing pathologists to recognize tumors that arose from the adrenal gland.[132] In 1908, two pathologists, Henri Alexais and Felix Peyron, introduced the scientific community to "paraganglioma" after they discovered extra-adrenal tissue that reacted to chromium salts, which mimicked the reaction of the adrenal medulla.[133] Just four years later, German pathologist Ludwig Pick coined the term "pheochromocytoma" after he observed the consistent color change in tumors associated with the adrenal medulla.[134] Many surgeons attempted to remove these tumors over the next decade, but their patients died intraoperatively from shock. In 1926, Charles Mayo (a founder of the Mayo Clinic) became the first physician to successfully excise a pheochromocytoma.[134] However, Mayo was likely unaware of the diagnosis prior to the operation. Not until 1929 was a pheochromocytoma recognized preoperatively.[135] Throughout the early 1900s, the operative mortality rate for a pheochromocytoma ranged from 30-45%. Retrospective series have postulated that these alarmingly high death rates were due to the lack of a pre-operative blockade with alpha and beta-adrenoceptor antagonist and the need for modern anesthesia practices.[136] From this point forward, physician-scientists have been recognizing patterns in patients with pheochromocytoma and identifying genetic associations and various syndromes.[135] EpidemiologyeditAccording to the North American Neuroendocrine Tumor Society, the prevalence of pheochromocytoma is between 1:2500 and 1:6500, meaning that for every 2,500 - 6,500 people, there is (on average) one person with pheochromocytoma.[137] In the United States, this equates to an annual incidence (new cases per year) of 500 to 1600 cases.[137] However, approximations in the early 2000's reported that upwards of 50% of pheochromocytoma diagnoses are at autopsy; therefore, the above estimations may be lower than expected.[9] In a 50-year autopsy case series, the Mayo Clinic reviewed 54 pheochromocytoma cases between 1928-1977 and discovered that just 24% of the patients were correctly diagnosed prior to their death.[138] Outside of the United States, several countries have documented their own epidemiological studies and compared them to what is known in North America. In the first national, epidemiological population-based study in Asia utilizing Korean National Health Insurance Service data, the prevalence of a pheochromocytoma was reported at 2.13 per 100,000 persons with an incidence of 0.18 per 100,000 person-years.[139] This is lower than the occurrence reported from Rochester, Minnesota (0.8 per 100,000 person-years) in a study conducted from 1950-1979.[140] However, the Netherlands also conducted a study using a nationwide registry and reported incidence results of 0.57 per 100,000 person-years from 2011-2015, which was a significant increase from their 0.37 cases per 100,000 person-years reported from 1995-1999.[141] Current hypotheses for why the incidence of pheochromocytoma is growing in the Dutch population point to the advent of modern imaging evaluation and the ability to detect these tumors prior to death.[142] While each of the above studies reported varying incidence and prevalence values, all have indicated that the average age at initial diagnosis is between the third to fifth decade of life.[143] When younger patients are diagnosed with a pheochromocytoma, there should be a high suspicion for hereditary disease, as genetic anticipation (earlier disease onset with each generation) is associated with some mutations.[144] 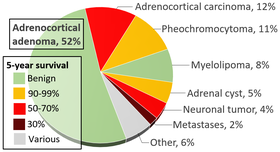 Classically, the pheochromocytoma "rules of 10" have been taught, particularly to medical students[145]:
Despite the prominence in many respected textbooks, these guidelines have since been established as inaccurate and should not be used in current epidemiological discussions.[143] As suggested above, incidental imaging has become a major player in the diagnosis of patients with pheochromocytoma, with current estimates between 10-49% of all cases diagnosed after imaging was obtained for another reason. When an adrenal nodule (potential tumor) is discovered on computed tomography or magnetic resonance imaging, there is between a 5 and 10% chance the lesion is a pheochromocytoma. [143] The incidence of adrenal tumors is found in the infographic above, with pheochromocytoma noted in yellow in the top right corner.
It mostly occurs in young or middle age adults, though it presents earlier in hereditary cases.
Society and CultureeditWhile a rare disease, there have been several references to pheochromocytoma in popular culture and the media, specifically medical television dramas. Additionally, there is a strong online patient advocacy community that works to connect patients with rare diseases and allows them to meet other individuals who are experiencing similar diagnoses and treatment strategies.  Zebra Culture In the medical community, students are often taught "when you hear hoofbeats, think horses, not zebras." [146] In other words, common diagnoses are common and you should first rule out what is most expected (the horses) before diving into the rare etiologies that are far less likely to be correct (the zebras). However, the symbol of the zebra has become increasingly powerful to members of the rare disease community and resulted in several organizations, societies, and special events (Rare Disease Day®) to celebrate when the answer is in fact, the least common option.[147] The National Organization for Rare Disorders is a United States-based advocacy parent organization with the goal of promoting awareness and research opportunities to cure rare diseases.[148] Groups such as these encourage patients to become their own advocates and change agents in their healthcare decision making processes. Support Groups and Recognition In 2006, the Pheochromocytoma and Paraganglioma Research Support Organization published a list of advocacy organizations specific to the pheochromocytoma community.[149] The two largest organizations (The Pheo-Para Alliance and PheoPara Troopers) have since combined forces and are now the most prominent support group.[150] In 2020, the inaugural Pheochromocytoma and Paraganglioma Awareness Week (August 23rd - August 29th) launched a social media campaign with videos, infographics, and memes to promote awareness and increase the general knowledge of these rare diseases. Other groups also include the Von Hippel-Lindau Family Alliance (VHL Alliance) which specifically provides information and support to patients with pheochromocytoma and a VHL genetic variant. [151] While not specific to pheochromocytoma, the Neuroendocrine Tumor Research Foundation is an avenue where patients can learn about the latest treatment options, research opportunities, and educational seminars.[152] Finally, the Rare Cancer Alliance is another foundation that targets the "zebra cancers" that are often not discussed in popular media.[153] In 2020, a pheochromocytoma patient in Albuquerque, New Mexico worked alongside local city officials and got Mayor Timothy M. Keller to officially proclaim August 23rd as "Pheo Para Awareness Day." Media  In July of 2012, an actual pheochromocytoma patient, Tannis Brown, former Vice-President of the PheoPara Troopers, was featured on the Discovery Fit & Health Network program Diagnosis: Dead or Alive. [154]The show highlighted her personal struggle with misdiagnosed disease as many physicians felt her episodic headaches and hypertension (high blood pressure) were related to stress.[155] Apart from featuring real-life stories of pheochromocytoma patients, this rare tumor has also been featured on primetime medical television dramas as well. In the opening episode of the second season (S2: E1, Acceptance) of House, Dr. Gregory House (Hugh Laurie) consults on a death row inmate who is later discovered to have pheochromocytoma after he experiences unexplainable fits of rage that ended in murder.[156] However, this depiction of a pheochromocytoma patient is factually inaccurate and disturbing. The "adrenaline rush" felt in patients with functionally active tumors is unlikely (if ever) to have been reported to random bouts of violence. In the seventh and eighth seasons of Greys Anatomy, series regular Dr. Teddy Altman (Kim Raver) finds herself married to a patient (Henry Burton, actor Scott Foley) due to his lack of medical insurance.[157] It is later revealed that he has a Von Hippel-Lindau (VHL) mutation that has resulted in a rare adrenal tumor known as pheochromocytoma. The several series arc was met with varying opinions from the rare disease community.[158] Then executive Director of the VHL Alliance was happy with the portrayal of a VHL patient in mainstream media, but pointed out that of the four scripts she knew of with a VHL patient, three involved a pheochromocytoma, which actually occurs in less than a fifth of all VHL patients.[159][160] While no patient is actually featured in the episode, in the premier season of Scrubs, the resident physicians are questioned on rounds about the initial test of choice for a pheochromocytoma and the correct pre-operative treatment (which they incorrectly answered as an angiotensin-converting enzyme (ACE) inhibitor, but should be an alpha-adrenoceptor blocker).[161] Finally, there are many personal stories shared on YouTube of patient journeys with this rare neuroendocrine tumor.
|
- ^ a b Mercado-Asis, Leilani B.; Wolf, Katherine I.; Jochmanova, Ivana; Taïeb, David (2018-01). "PHEOCHROMOCYTOMA: A GENETIC AND DIAGNOSTIC UPDATE". Endocrine Practice: Official Journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 24 (1): 78–90. doi:10.4158/EP-2017-0057. ISSN 1530-891X. PMID 29144820.
{{cite journal}}: Check date values in:|date=(help) - ^ Wolf, Katherine I.; Jha, Abhishek; van Berkel, Anouk; Wild, Damian; Janssen, Ingo; Millo, Corina M.; Janssen, M. J. R.; Gonzales, Melissa K.; Timmers, Henri J. K. M.; Pacak, Karel (2019-06). "Eruption of Metastatic Paraganglioma After Successful Therapy with 177Lu/90Y-DOTATOC and 177Lu-DOTATATE". Nuclear Medicine and Molecular Imaging. 53 (3): 223–230. doi:10.1007/s13139-019-00579-w. ISSN 1869-3474. PMC 6554376. PMID 31231443.
{{cite journal}}: Check date values in:|date=(help) - ^ Oyasu, Ryoichi; Yang, Ximing J.; Yoshida, Osamu, eds. (2008), "What is the difference between pheochromocytoma and paraganglioma? What are the familial syndromes that have pheochromocytoma as a component? What are the pathologic features of pheochromocytoma indicating malignancy?", Questions in Daily Urologic Practice: Updates for Urologists and Diagnostic Pathologists, Tokyo: Springer Japan, pp. 280–284, doi:10.1007/978-4-431-72819-1_49, ISBN 978-4-431-72819-1, retrieved 2020-08-09
- ^ a b Lenders, Jacques W. M.; Pacak, Karel; Walther, McClellan M.; Linehan, W. Marston; Mannelli, Massimo; Friberg, Peter; Keiser, Harry R.; Goldstein, David S.; Eisenhofer, Graeme (2002-03-20). "Biochemical diagnosis of pheochromocytoma: which test is best?". JAMA. 287 (11): 1427–1434. doi:10.1001/jama.287.11.1427. ISSN 0098-7484. PMID 11903030.
- ^ Moore, Michael G.; Netterville, James L.; Mendenhall, William M.; Isaacson, Brandon; Nussenbaum, Brian (2016-04). "Head and Neck Paragangliomas: An Update on Evaluation and Management". Otolaryngology--Head and Neck Surgery: Official Journal of American Academy of Otolaryngology-Head and Neck Surgery. 154 (4): 597–605. doi:10.1177/0194599815627667. ISSN 1097-6817. PMID 26861230.
{{cite journal}}: Check date values in:|date=(help) - ^ Williams, Michelle D (2017-03-20). "Paragangliomas of the Head and Neck: An Overview from Diagnosis to Genetics". Head and Neck Pathology. 11 (3): 278–287. doi:10.1007/s12105-017-0803-4. ISSN 1936-055X. PMC 5550402. PMID 28321772.
- ^ Tevosian, Sergei G.; Ghayee, Hans K. (12 2019). "Pheochromocytomas and Paragangliomas". Endocrinology and Metabolism Clinics of North America. 48 (4): 727–750. doi:10.1016/j.ecl.2019.08.006. ISSN 1558-4410. PMID 31655773.
{{cite journal}}: Check date values in:|date=(help) - ^ Zuber, Samuel M.; Kantorovich, Vitaly; Pacak, Karel (2011-6). "Hypertension in Pheochromocytoma: Characteristics and Treatment". Endocrinology and metabolism clinics of North America. 40 (2): 295–311. doi:10.1016/j.ecl.2011.02.002. ISSN 0889-8529. PMC 3094542. PMID 21565668.
{{cite journal}}: Check date values in:|date=(help) - ^ a b c d Manger, William M. (2006-08). "An overview of pheochromocytoma: history, current concepts, vagaries, and diagnostic challenges". Annals of the New York Academy of Sciences. 1073: 1–20. doi:10.1196/annals.1353.001. ISSN 0077-8923. PMID 17102067.
{{cite journal}}: Check date values in:|date=(help) - ^ Lanier, Jeffrey B.; Mote, Matthew B.; Clay, Emily C. (2011-09-01). "Evaluation and management of orthostatic hypotension". American Family Physician. 84 (5): 527–536. ISSN 1532-0650. PMID 21888303.
- ^ Mitchell, Louisa; Bellis, Fionn (2007-09). "Phaeochromocytoma--"the great mimic": an unusual presentation". Emergency medicine journal: EMJ. 24 (9): 672–673. doi:10.1136/emj.2007.049569. ISSN 1472-0213. PMC 2464664. PMID 17711956.
{{cite journal}}: Check date values in:|date=(help) - ^ Harrison's principles of internal medicine. Braunwald, Eugene, 1929- (15th ed. ed.). New York: McGraw-Hill. 2001. ISBN 0-07-913686-9. OCLC 44860874.
{{cite book}}:|edition=has extra text (help)CS1 maint: others (link) - ^ Prejbisz, Aleksander; Lenders, Jacques W. M.; Eisenhofer, Graeme; Januszewicz, Andrzej (2011-11). "Cardiovascular manifestations of phaeochromocytoma". Journal of Hypertension. 29 (11): 2049–2060. doi:10.1097/HJH.0b013e32834a4ce9. ISSN 1473-5598. PMID 21826022.
{{cite journal}}: Check date values in:|date=(help) - ^ Young, William F. (2007-12). "Adrenal causes of hypertension: pheochromocytoma and primary aldosteronism". Reviews in Endocrine & Metabolic Disorders. 8 (4): 309–320. doi:10.1007/s11154-007-9055-z. ISSN 1389-9155. PMID 17914676.
{{cite journal}}: Check date values in:|date=(help) - ^ Liao, W. B.; Liu, C. F.; Chiang, C. W.; Kung, C. T.; Lee, C. W. (2000-09). "Cardiovascular manifestations of pheochromocytoma". The American Journal of Emergency Medicine. 18 (5): 622–625. doi:10.1053/ajem.2000.7341. ISSN 0735-6757. PMID 10999582.
{{cite journal}}: Check date values in:|date=(help) - ^ Young, William F. (2007-12). "Adrenal causes of hypertension: pheochromocytoma and primary aldosteronism". Reviews in Endocrine & Metabolic Disorders. 8 (4): 309–320. doi:10.1007/s11154-007-9055-z. ISSN 1389-9155. PMID 17914676.
{{cite journal}}: Check date values in:|date=(help) - ^ a b c Prejbisz, Aleksander; Lenders, Jacques W. M.; Eisenhofer, Graeme; Januszewicz, Andrzej (2011-11). "Cardiovascular manifestations of phaeochromocytoma". Journal of Hypertension. 29 (11): 2049–2060. doi:10.1097/HJH.0b013e32834a4ce9. ISSN 1473-5598. PMID 21826022.
{{cite journal}}: Check date values in:|date=(help) - ^ Liao, W. B.; Liu, C. F.; Chiang, C. W.; Kung, C. T.; Lee, C. W. (2000-09). "Cardiovascular manifestations of pheochromocytoma". The American Journal of Emergency Medicine. 18 (5): 622–625. doi:10.1053/ajem.2000.7341. ISSN 0735-6757. PMID 10999582.
{{cite journal}}: Check date values in:|date=(help) - ^ a b Zhang, Rong; Gupta, Deepashree; Albert, Stewart G. (2017-12-15). "Pheochromocytoma as a reversible cause of cardiomyopathy: Analysis and review of the literature". International Journal of Cardiology. 249: 319–323. doi:10.1016/j.ijcard.2017.07.014. ISSN 1874-1754. PMID 29121733.
- ^ a b Agrawal, Sahil; Shirani, Jamshid; Garg, Lohit; Singh, Amitoj; Longo, Santo; Longo, Angelita; Fegley, Mark; Stone, Lauren; Razavi, Muhammad; Radoianu, Nicoleta; Nanda, Sudip (2017-03-26). "Pheochromocytoma and stress cardiomyopathy: Insight into pathogenesis". World Journal of Cardiology. 9 (3): 255–260. doi:10.4330/wjc.v9.i3.255. ISSN 1949-8462. PMC 5368675. PMID 28400922.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Van, Yang-Hau; Wang, Huei-Shyong; Lai, Ching-Horng; Lin, Jer-Nan; Lo, Fu-Sung (2002-11). "Pheochromocytoma presenting as stroke in two Taiwanese children". Journal of pediatric endocrinology & metabolism: JPEM. 15 (9): 1563–1567. doi:10.1515/jpem.2002.15.9.1563. ISSN 0334-018X. PMID 12503867.
{{cite journal}}: Check date values in:|date=(help) - ^ Abourazzak, Sana; Atmani, Samir; Arqam, Larbi El; Chaouki, Sanae; Labib, Smail; Harrandou, Moustapha; Tizniti, Siham; Bouabdellah, Youssef; Bouharrou, Abdelhak; Hida, Moustapha (2010-05-11). "Cerebral ischaemic stroke and bilateral pheochromocytoma". BMJ case reports. 2010. doi:10.1136/bcr.12.2009.2535. ISSN 1757-790X. PMC 3047554. PMID 22736758.
- ^ DAGARTZIKAS, MARIA I.; SPRAGUE, KELLY; CARTER, GUY; TOBIAS, JOSEPH D. (2002-02). "Cerebrovascular event, dilated cardiomyopathy, and pheochromocytoma". Pediatric Emergency Care. 18 (1): 33–35. doi:10.1097/00006565-200202000-00011. ISSN 0749-5161.
{{cite journal}}: Check date values in:|date=(help) - ^ Cohen, Jenny K.; Cisco, Robin M.; Scholten, Anouk; Mitmaker, Elliot; Duh, Quan-Yang (2014-04). "Pheochromocytoma crisis resulting in acute heart failure and cardioembolic stroke in a 37-year-old man". Surgery. 155 (4): 726–727. doi:10.1016/j.surg.2012.11.013. ISSN 0039-6060.
{{cite journal}}: Check date values in:|date=(help) - ^ Lin, Pi Chi Hsu, Jen Te Chung, Chang Min Chang, Shih Tai. Pheochromocytoma Underlying Hypertension, Stroke, and Dilated Cardiomyopathy. OCLC 679006463.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Buchbinder, Neil A.; Yu, Run; Rosenbloom, Barry E.; Sherman, C. T.; Silberman, Allan W. (2009-12). "Left Ventricular Thrombus and Embolic Stroke Caused by a Functional Paraganglioma". The Journal of Clinical Hypertension. 11 (12): 734–737. doi:10.1111/j.1751-7176.2009.00182.x. ISSN 1524-6175.
{{cite journal}}: Check date values in:|date=(help) - ^ Luiz, H. V.; da Silva, T. N.; Pereira, B. D.; Santos, J. G.; Goncalves, D.; Manita, I.; Portugal, J. (2013-11-25). "Malignant Paraganglioma Presenting With Hemorrhagic Stroke in a Child". PEDIATRICS. 132 (6): e1709–e1714. doi:10.1542/peds.2013-0492. ISSN 0031-4005.
- ^ Potapova, G.; Chazova, I.; Kuznetsov, N.; Sitina, V.; Popov, E.; Gavrilov, I. (2011-06). "PHEOCHROMOCYTOMA AND STROKE: PP.37.255". Journal of Hypertension. 29: e505. doi:10.1097/00004872-201106001-01534. ISSN 0263-6352.
{{cite journal}}: Check date values in:|date=(help) - ^ Anderson, N. E.; Chung, K.; Willoughby, E.; Croxson, M. S. (2012-12-01). "Neurological manifestations of phaeochromocytomas and secretory paragangliomas: a reappraisal". Journal of Neurology, Neurosurgery & Psychiatry. 84 (4): 452–457. doi:10.1136/jnnp-2012-303028. ISSN 0022-3050.
- ^ Shemin, Douglas (1990-11-01). "Pheochromocytoma Presenting as Rhabdomyolysis and Acute Myoglobinuric Renal Failure". Archives of Internal Medicine. 150 (11): 2384. doi:10.1001/archinte.1990.00390220118024. ISSN 0003-9926.
- ^ Hamada, N.; Akamatsu, A.; Joh, T. (1993-01). "A case of pheochromocytoma complicated with acute renal failure and cardiomyopathy". Japanese Circulation Journal. 57 (1): 84–90. doi:10.1253/jcj.57.84. ISSN 0047-1828. PMID 8437346.
{{cite journal}}: Check date values in:|date=(help) - ^ Celik, Huseyin; Celik, Ozlem; Guldiken, Sibel; Inal, Volkan; Puyan, Fulya Oz; Tugrul, Armagan (2014-02). "Pheochromocytoma presenting with rhabdomyolysis and acute renal failure: a case report". Renal Failure. 36 (1): 104–107. doi:10.3109/0886022X.2013.832856. ISSN 1525-6049. PMID 24059440.
{{cite journal}}: Check date values in:|date=(help) - ^ Takabatake, T.; Kawabata, M.; Ohta, H.; Yamamoto, Y.; Ishida, Y.; Hara, H.; Hattori, N. (1985-07). "Acute renal failure and transient, massive proteinuria in a case of pheochromocytoma". Clinical Nephrology. 24 (1): 47–49. ISSN 0301-0430. PMID 4017298.
{{cite journal}}: Check date values in:|date=(help) - ^ Lorz, W.; Cottier, Chr; Imhof, E.; Gyr, N. (1993). "Multiple organ failure and coma as initial presentation of pheochromocytoma in a patient with multiple endocrine neoplasia (MEN) type II A". Intensive Care Medicine. 19 (4): 235–238. doi:10.1007/BF01694777. ISSN 0342-4642. PMC 7095150. PMID 8103532.
- ^ Marshall, John C. (2001). The multiple organ dysfunction syndrome. Zuckschwerdt.
- ^ Newell, K. A.; Prinz, R. A.; Pickleman, J.; Braithwaite, S.; Brooks, M.; Karson, T. H.; Glisson, S. (1988-08). "Pheochromocytoma multisystem crisis. A surgical emergency". Archives of Surgery (Chicago, Ill.: 1960). 123 (8): 956–959. doi:10.1001/archsurg.1988.01400320042007. ISSN 0004-0010. PMID 2899426.
{{cite journal}}: Check date values in:|date=(help) - ^ Histed, Stephanie N.; Lindenberg, Maria L.; Mena, Esther; Turkbey, Baris; Choyke, Peter L.; Kurdziel, Karen A. (2012-4). "Review of Functional/ Anatomic Imaging in Oncology". Nuclear Medicine Communications. 33 (4): 349–361. doi:10.1097/MNM.0b013e32834ec8a5. ISSN 0143-3636. PMC 3295905. PMID 22314804.
{{cite journal}}: Check date values in:|date=(help) - ^ Neumann, Hartmut P.H.; Young, William F.; Eng, Charis (2019-08-08). Longo, Dan L. (ed.). "Pheochromocytoma and Paraganglioma". New England Journal of Medicine. 381 (6): 552–565. doi:10.1056/NEJMra1806651. ISSN 0028-4793.
- ^ a b Timmers, H.J.L.; Taieb, D.; Pacak, K. (2012-03-07). "Current and Future Anatomical and Functional Imaging Approaches to Pheochromocytoma and Paraganglioma". Hormone and Metabolic Research. 44 (05): 367–372. doi:10.1055/s-0031-1299712. ISSN 0018-5043.
- ^ McCullough, Peter A.; Choi, James P.; Feghali, Georges A.; Schussler, Jeffrey M.; Stoler, Robert M.; Vallabahn, Ravi C.; Mehta, Ankit (09 27, 2016). "Contrast-Induced Acute Kidney Injury". Journal of the American College of Cardiology. 68 (13): 1465–1473. doi:10.1016/j.jacc.2016.05.099. ISSN 1558-3597. PMID 27659469.
{{cite journal}}: Check date values in:|date=(help) - ^ Caraiani, Cosmin; Dong, Yi; Rudd, Anthony G.; Dietrich, Christoph F. (2018-12-08). "Reasons for inadequate or incomplete imaging techniques". Medical Ultrasonography. 20 (4): 498–507. doi:10.11152/mu-1736. ISSN 2066-8643. PMID 30534659.
- ^ a b Castinetti, Frédéric; Kroiss, Alexander; Kumar, Rakesh; Pacak, Karel; Taieb, David (2015-08-01). "15 YEARS OF PARAGANGLIOMA: Imaging and imaging-based treatment of pheochromocytoma and paraganglioma". Endocrine-Related Cancer. 22 (4): T135–T145. doi:10.1530/ERC-15-0175. ISSN 1351-0088.
- ^ Leung, Katherine; Stamm, Michael; Raja, Asim; Low, Gavin (2013-02). "Pheochromocytoma: the range of appearances on ultrasound, CT, MRI, and functional imaging". AJR. American journal of roentgenology. 200 (2): 370–378. doi:10.2214/AJR.12.9126. ISSN 1546-3141. PMID 23345359.
{{cite journal}}: Check date values in:|date=(help) - ^ Chaudhary, Vikas; Bano, Shahina (2012). "Anatomical and functional imaging in endocrine hypertension". Indian Journal of Endocrinology and Metabolism. 16 (5): 713. doi:10.4103/2230-8210.100659. ISSN 2230-8210.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Rufini, Vittoria; Treglia, Giorgio; Perotti, Germano; Giordano, Alessandro (2013-01). "The evolution in the use of MIBG scintigraphy in pheochromocytomas and paragangliomas". Hormones. 12 (1): 58–68. doi:10.1007/bf03401287. ISSN 1109-3099.
{{cite journal}}: Check date values in:|date=(help) - ^ van Hulsteijn, L. T.; Niemeijer, N. D.; Dekkers, O. M.; Corssmit, E. P. M. (2014-04). "(131)I-MIBG therapy for malignant paraganglioma and phaeochromocytoma: systematic review and meta-analysis". Clinical Endocrinology. 80 (4): 487–501. doi:10.1111/cen.12341. ISSN 1365-2265. PMID 24118038.
{{cite journal}}: Check date values in:|date=(help) - ^ a b c Lenders, Jacques W. M.; Eisenhofer, Graeme (2017-06). "Update on Modern Management of Pheochromocytoma and Paraganglioma". Endocrinology and Metabolism (Seoul, Korea). 32 (2): 152–161. doi:10.3803/EnM.2017.32.2.152. ISSN 2093-596X. PMC 5503859. PMID 28685506.
{{cite journal}}: Check date values in:|date=(help) - ^ a b Timmers, Henri J. L. M.; Chen, Clara C.; Carrasquillo, Jorge A.; Whatley, Millie; Ling, Alexander; Eisenhofer, Graeme; King, Kathryn S.; Rao, Jyotsna U.; Wesley, Robert A.; Adams, Karen T.; Pacak, Karel (2012-05-02). "Staging and functional characterization of pheochromocytoma and paraganglioma by 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography". Journal of the National Cancer Institute. 104 (9): 700–708. doi:10.1093/jnci/djs188. ISSN 1460-2105. PMC 3341309. PMID 22517990.
- ^ Taïeb, D. Tessonnier, L. Sebag, F. Niccoli-Sire, P. Morange, I. Colavolpe, C. De Micco, C. Barlier, A. Palazzo, F. F. Henry, J. F. Mundler, O. The role of 18F-FDOPA and 18F-FDG-PET in the management of malignant and multifocal phaeochromocytomas. OCLC 798350389.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Taïeb, David; Hicks, Rodney J.; Hindié, Elif; Guillet, Benjamin A.; Avram, Anca; Ghedini, Pietro; Timmers, Henri J.; Scott, Aaron T.; Elojeimy, Saeed; Rubello, Domenico; Virgolini, Irène J. (2019-06-29). "European Association of Nuclear Medicine Practice Guideline/Society of Nuclear Medicine and Molecular Imaging Procedure Standard 2019 for radionuclide imaging of phaeochromocytoma and paraganglioma". European Journal of Nuclear Medicine and Molecular Imaging. 46 (10): 2112–2137. doi:10.1007/s00259-019-04398-1. ISSN 1619-7070.
- ^ Jha, Abhishek; Ling, Alexander; Millo, Corina; Gupta, Garima; Viana, Bruna; Lin, Frank I.; Herscovitch, Peter; Adams, Karen T.; Taïeb, David; Metwalli, Adam R.; Linehan, W. Marston (05 2018). "Superiority of 68Ga-DOTATATE over 18F-FDG and anatomic imaging in the detection of succinate dehydrogenase mutation (SDHx )-related pheochromocytoma and paraganglioma in the pediatric population". European Journal of Nuclear Medicine and Molecular Imaging. 45 (5): 787–797. doi:10.1007/s00259-017-3896-9. ISSN 1619-7089. PMC 6707509. PMID 29204718.
{{cite journal}}: Check date values in:|date=(help) - ^ Janssen, Ingo; Chen, Clara C.; Millo, Corina M.; Ling, Alexander; Taieb, David; Lin, Frank I.; Adams, Karen T.; Wolf, Katherine I.; Herscovitch, Peter; Fojo, Antonio T.; Buchmann, Inga (2016-09). "PET/CT comparing (68)Ga-DOTATATE and other radiopharmaceuticals and in comparison with CT/MRI for the localization of sporadic metastatic pheochromocytoma and paraganglioma". European Journal of Nuclear Medicine and Molecular Imaging. 43 (10): 1784–1791. doi:10.1007/s00259-016-3357-x. ISSN 1619-7089. PMID 26996779.
{{cite journal}}: Check date values in:|date=(help) - ^ Kroiss, A. S.; Uprimny, C.; Shulkin, B. L.; Gruber, L.; Frech, A.; Jazbec, T.; Girod, P. P.; Url, C.; Thomé, C.; Riechelmann, H.; Sprinzl, G. M. (2019-03). "68Ga-DOTATOC PET/CT in the localization of metastatic extra-adrenal paraganglioma and pheochromocytoma compared with 18F-DOPA PET/CT". Revista Espanola De Medicina Nuclear E Imagen Molecular. 38 (2): 94–99. doi:10.1016/j.remn.2018.09.004. ISSN 2253-8070. PMID 30630744.
{{cite journal}}: Check date values in:|date=(help) - ^ a b Wiseman, Douglas; Lakis, Mustapha El; Nilubol, Naris (2019-07). "Precision Surgery for Pheochromocytomas and Paragangliomas". Hormone and Metabolic Research = Hormon- Und Stoffwechselforschung = Hormones Et Metabolisme. 51 (7): 470–482. doi:10.1055/a-0926-3618. ISSN 1439-4286. PMID 31307109.
{{cite journal}}: Check date values in:|date=(help) - ^ a b Lenders, Jacques W. M.; Duh, Quan-Yang; Eisenhofer, Graeme; Gimenez-Roqueplo, Anne-Paule; Grebe, Stefan K. G.; Murad, Mohammad Hassan; Naruse, Mitsuhide; Pacak, Karel; Young, William F. (2014-06-01). "Pheochromocytoma and Paraganglioma: An Endocrine Society Clinical Practice Guideline". The Journal of Clinical Endocrinology & Metabolism. 99 (6): 1915–1942. doi:10.1210/jc.2014-1498. ISSN 0021-972X.
- ^ Aggeli, Chrysanthi; Nixon, Alexander M.; Parianos, Christos; Vletsis, Georgios; Papanastasiou, Labrini; Markou, Athina; Kounadi, Theodora; Piaditis, Georgrios; Zografos, Georgios N. (2017-10). "Surgery for pheochromocytoma: A 20-year experience of a single institution". Hormones (Athens, Greece). 16 (4): 388–395. doi:10.14310/horm.2002.1759. ISSN 2520-8721. PMID 29518759.
{{cite journal}}: Check date values in:|date=(help) - ^ Goffredo, P.; Adam, M. A.; Thomas, S. M.; Scheri, R. P.; Sosa, J. A.; Roman, S. A. (2015-08). "Patterns of Use and Short-Term Outcomes of Minimally Invasive Surgery for Malignant Pheochromocytoma: A Population-Level Study". World Journal of Surgery. 39 (8): 1966–1973. doi:10.1007/s00268-015-3040-6. ISSN 1432-2323. PMID 25821949.
{{cite journal}}: Check date values in:|date=(help) - ^ Berber, Eren; Mitchell, Jamie; Milas, Mira; Siperstein, Allan (2010-08). "Robotic posterior retroperitoneal adrenalectomy: operative technique". Archives of Surgery (Chicago, Ill.: 1960). 145 (8): 781–784. doi:10.1001/archsurg.2010.148. ISSN 1538-3644. PMID 20713932.
{{cite journal}}: Check date values in:|date=(help) - ^ Aliyev, Shamil; Karabulut, Koray; Agcaoglu, Orhan; Wolf, Katherine; Mitchell, Jamie; Siperstein, Allan; Berber, Eren (2013-12). "Robotic versus laparoscopic adrenalectomy for pheochromocytoma". Annals of Surgical Oncology. 20 (13): 4190–4194. doi:10.1245/s10434-013-3134-z. ISSN 1534-4681. PMID 23864309.
{{cite journal}}: Check date values in:|date=(help) - ^ a b Neumann, Hartmut P. H.; Tsoy, Uliana; Bancos, Irina; Amodru, Vincent; Walz, Martin K.; Tirosh, Amit; Kaur, Ravinder Jeet; McKenzie, Travis; Qi, Xiaoping; Bandgar, Tushar; Petrov, Roman (08 02, 2019). "Comparison of Pheochromocytoma-Specific Morbidity and Mortality Among Adults With Bilateral Pheochromocytomas Undergoing Total Adrenalectomy vs Cortical-Sparing Adrenalectomy". JAMA network open. 2 (8): e198898. doi:10.1001/jamanetworkopen.2019.8898. ISSN 2574-3805. PMC 6692838. PMID 31397861.
{{cite journal}}: Check date values in:|date=(help) - ^ Lee, Jeffrey E.; Curley, Steven A.; Gagel, Robert F.; Evans, Douglas B.; Hickey, Robert C. (1996-12-01). "Cortical-sparing adrenalectomy for patients with bilateral pheochromocytoma". Surgery. 120 (6): 1064–1071. doi:10.1016/S0039-6060(96)80056-0. ISSN 0039-6060. PMID 8957496.
- ^ a b c d e f Pacak, Karel (2007-11). "Preoperative management of the pheochromocytoma patient". The Journal of Clinical Endocrinology and Metabolism. 92 (11): 4069–4079. doi:10.1210/jc.2007-1720. ISSN 0021-972X. PMID 17989126.
{{cite journal}}: Check date values in:|date=(help) - ^ Wolf, Katherine I.; Santos, Jenn Rachelle U.; Pacak, Karel (2019-01). "WHY TAKE THE RISK? WE ONLY LIVE ONCE: THE DANGERS ASSOCIATED WITH NEGLECTING A PRE-OPERATIVE ALPHA ADRENOCEPTOR BLOCKADE IN PHEOCHROMOCYTOMA PATIENTS". Endocrine Practice: Official Journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 25 (1): 106–108. doi:10.4158/EP-2018-0455. ISSN 1530-891X. PMC 6478021. PMID 30289301.
{{cite journal}}: Check date values in:|date=(help) - ^ Graham, R. M.; Thornell, I. R.; Gain, J. M.; Bagnoli, C.; Oates, H. F.; Stokes, G. S. (1976-11-27). "Prazosin: the first-dose phenomenon". British Medical Journal. 2 (6047): 1293–1294. doi:10.1136/bmj.2.6047.1293. ISSN 0007-1447. PMC 1689975. PMID 793676.
- ^ Kleeman, F. J. (1977-06). "Phenoxybenzamine". The Journal of Urology. 117 (6): 814. doi:10.1016/s0022-5347(17)58643-7. ISSN 0022-5347. PMID 875171.
{{cite journal}}: Check date values in:|date=(help) - ^ Brunaud, Laurent; Boutami, Myriam; Nguyen-Thi, Phi-Linh; Finnerty, Brendan; Germain, Adeline; Weryha, Georges; Fahey, Thomas J.; Mirallie, Eric; Bresler, Laurent; Zarnegar, Rasa (2014-12). "Both preoperative alpha and calcium channel blockade impact intraoperative hemodynamic stability similarly in the management of pheochromocytoma". Surgery. 156 (6): 1410–1417, discussion1417–1418. doi:10.1016/j.surg.2014.08.022. ISSN 1532-7361. PMID 25456922.
{{cite journal}}: Check date values in:|date=(help) - ^ Clark, Barbara K. (1992-05-01). "Beta-adrenergic Blocking Agents: Their Current Status". AACN Advanced Critical Care. 3 (2): 447–460. doi:10.4037/15597768-1992-2016. ISSN 1559-7768.
- ^ Luiz, Henrique Vara; Tanchee, Mary Jane; Pavlatou, Maria G.; Yu, Run; Nambuba, Joan; Wolf, Katherine; Prodanov, Tamara; Wesley, Robert; Adams, Karen; Fojo, Tito; Pacak, Karel (07 2016). "Are patients with hormonally functional phaeochromocytoma and paraganglioma initially receiving a proper adrenoceptor blockade? A retrospective cohort study". Clinical Endocrinology. 85 (1): 62–69. doi:10.1111/cen.13066. ISSN 1365-2265. PMC 4899243. PMID 26998836.
{{cite journal}}: Check date values in:|date=(help) - ^ Sheaves, R.; Chew, S. L.; Grossman, A. B. (1995-01). "The dangers of unopposed beta-adrenergic blockade in phaeochromocytoma". Postgraduate Medical Journal. 71 (831): 58–59. doi:10.1136/pgmj.71.831.58-a. ISSN 0032-5473. PMC 2397901. PMID 7708599.
{{cite journal}}: Check date values in:|date=(help) - ^ van Brummelen, P.; Jie, K.; van Zwieten, P. A. (1986). "α-Adrenergic receptors in human blood vessels". British Journal of Clinical Pharmacology. 21 (Suppl 1): 33S–39S. ISSN 0306-5251. PMC 1400759. PMID 2871855.
- ^ Chruscinski, A.; Brede, M. E.; Meinel, L.; Lohse, M. J.; Kobilka, B. K.; Hein, L. (2001-11). "Differential distribution of beta-adrenergic receptor subtypes in blood vessels of knockout mice lacking beta(1)- or beta(2)-adrenergic receptors". Molecular Pharmacology. 60 (5): 955–962. doi:10.1124/mol.60.5.955. ISSN 0026-895X. PMID 11641423.
{{cite journal}}: Check date values in:|date=(help) - ^ a b Groeben, H.; Nottebaum, B. J.; Alesina, P. F.; Traut, A.; Neumann, H. P.; Walz, M. K. (2017-02). "Perioperative α-receptor blockade in phaeochromocytoma surgery: an observational case series". British Journal of Anaesthesia. 118 (2): 182–189. doi:10.1093/bja/aew392. ISSN 1471-6771. PMID 28100521.
{{cite journal}}: Check date values in:|date=(help) - ^ Lentschener, Claude; Baillard, Christophe; Dousset, Bertrand; Gaujoux, Sebastien (2019-02). "DOGMA IS MADE TO BE BROKEN. WHY ARE WE POSTPONING CURATIVE SURGERY TO ADMINISTER INEFFECTIVE ALPHA ADRENORECEPTOR BLOCKADE IN MOST PATIENTS UNDERGOING PHEOCHROMOCYTOMA REMOVAL?". Endocrine Practice: Official Journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 25 (2): 199. doi:10.4158/1934-2403-25.2.199. ISSN 1530-891X. PMID 30817194.
{{cite journal}}: Check date values in:|date=(help) - ^ Wolf, Katherine I.; Santos, Jenn Rachelle U.; Pacak, Karel (2019-01). "WHY TAKE THE RISK? WE ONLY LIVE ONCE: THE DANGERS ASSOCIATED WITH NEGLECTING A PRE-OPERATIVE ALPHA ADRENOCEPTOR BLOCKADE IN PHEOCHROMOCYTOMA PATIENTS". Endocrine Practice: Official Journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 25 (1): 106–108. doi:10.4158/EP-2018-0455. ISSN 1530-891X. PMC 6478021. PMID 30289301.
{{cite journal}}: Check date values in:|date=(help) - ^ Santos, Jenn Rachelle U.; Wolf, Katherine I.; Pacak, Karel (02 2019). "A NECESSITY, NOT A SECOND THOUGHT: PRE-OPERATIVE ALPHA-ADRENOCEPTOR BLOCKADE IN PHEOCHROMOCYTOMA PATIENTS". Endocrine Practice: Official Journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 25 (2): 200–201. doi:10.4158/1934-2403-25.2.200. ISSN 1530-891X. PMID 30817195.
{{cite journal}}: Check date values in:|date=(help) - ^ Challis, B. G.; Casey, R. T.; Simpson, H. L.; Gurnell, M. (2017-02). "Is there an optimal preoperative management strategy for phaeochromocytoma/paraganglioma?". Clinical Endocrinology. 86 (2): 163–167. doi:10.1111/cen.13252. ISSN 1365-2265. PMID 27696513.
{{cite journal}}: Check date values in:|date=(help) - ^ Jiang, Minchun; Ding, Huanyu; Liang, Ying; Tang, Juying; Lin, Ying; Xiang, Kexu; Guo, Ying; Zhang, Shaoling (03 2018). "Preoperative risk factors for haemodynamic instability during pheochromocytoma surgery in Chinese patients". Clinical Endocrinology. 88 (3): 498–505. doi:10.1111/cen.13544. ISSN 1365-2265. PMID 29292527.
{{cite journal}}: Check date values in:|date=(help) - ^ LENTSCHENER, C.; GAUJOUX, S.; THILLOIS, J. M.; DUBOC, D.; BERTHERAT, J.; OZIER, Y.; DOUSSET, B. (2009-04). "Increased arterial pressure is not predictive of haemodynamic instability in patients undergoing adrenalectomy for phaeochromocytoma". Acta Anaesthesiologica Scandinavica. 53 (4): 522–527. doi:10.1111/j.1399-6576.2008.01894.x. ISSN 0001-5172.
{{cite journal}}: Check date values in:|date=(help) - ^ a b Wong, C.; Yu, R. (2010-07). "Preoperative preparation for pheochromocytoma resection: physician survey and clinical practice". Experimental and Clinical Endocrinology & Diabetes: Official Journal, German Society of Endocrinology [and] German Diabetes Association. 118 (7): 400–404. doi:10.1055/s-0029-1225339. ISSN 1439-3646. PMID 19609840.
{{cite journal}}: Check date values in:|date=(help) - ^ a b c d Mamilla, Divya; Araque, Katherine A.; Brofferio, Alessandra; Gonzales, Melissa K.; Sullivan, James N.; Nilubol, Naris; Pacak, Karel (07 03, 2019). "Postoperative Management in Patients with Pheochromocytoma and Paraganglioma". Cancers. 11 (7). doi:10.3390/cancers11070936. ISSN 2072-6694. PMC 6678461. PMID 31277296.
{{cite journal}}: Check date values in:|date=(help)CS1 maint: unflagged free DOI (link) - ^ Naranjo, Julian; Dodd, Sarah; Martin, Yvette N. (2017-08). "Perioperative Management of Pheochromocytoma". Journal of Cardiothoracic and Vascular Anesthesia. 31 (4): 1427–1439. doi:10.1053/j.jvca.2017.02.023. ISSN 1532-8422. PMID 28392094.
{{cite journal}}: Check date values in:|date=(help) - ^ Aronow, Wilbert S. (2017-05). "Treatment of hypertensive emergencies". Annals of Translational Medicine. 5 (S1): S5–S5. doi:10.21037/atm.2017.03.34. PMC 5440310. PMID 28567387.
{{cite journal}}: Check date values in:|date=(help)CS1 maint: PMC format (link) CS1 maint: unflagged free DOI (link) - ^ Chiang, Han-Liang; Huang, Yu-Chi; Lin, Huey-Shyan; Chan, Min-Ho; Chia, Yuan-Yi (2019). "Hypertension and Postoperative Pain: A Prospective Observational Study". Pain Research & Management. 2019: 8946195. doi:10.1155/2019/8946195. ISSN 1918-1523. PMC 6343159. PMID 30728877.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Naranjo, Julian; Dodd, Sarah; Martin, Yvette N. (2017-08). "Perioperative Management of Pheochromocytoma". Journal of Cardiothoracic and Vascular Anesthesia. 31 (4): 1427–1439. doi:10.1053/j.jvca.2017.02.023. ISSN 1532-8422. PMID 28392094.
{{cite journal}}: Check date values in:|date=(help) - ^ Iqbal, Ahmed; Heller, Simon (06 2016). "Managing hypoglycaemia". Best Practice & Research. Clinical Endocrinology & Metabolism. 30 (3): 413–430. doi:10.1016/j.beem.2016.06.004. ISSN 1878-1594. PMID 27432075.
{{cite journal}}: Check date values in:|date=(help) - ^ Dungan, Kathleen; Merrill, Jennifer; Long, Clarine; Binkley, Philip (11 27, 2019). "Effect of beta blocker use and type on hypoglycemia risk among hospitalized insulin requiring patients". Cardiovascular Diabetology. 18 (1): 163. doi:10.1186/s12933-019-0967-1. ISSN 1475-2840. PMC 6882013. PMID 31775749.
{{cite journal}}: Check date values in:|date=(help)CS1 maint: unflagged free DOI (link) - ^ Shen, Wen T.; Lee, James; Kebebew, Electron; Clark, Orlo H.; Duh, Quan-Yang (2006-08). "Selective use of steroid replacement after adrenalectomy: lessons from 331 consecutive cases". Archives of Surgery (Chicago, Ill.: 1960). 141 (8): 771–774, discussion 774–776. doi:10.1001/archsurg.141.8.771. ISSN 0004-0010. PMID 16924084.
{{cite journal}}: Check date values in:|date=(help) - ^ a b MacKenzie, C. Ronald; Goodman, Susan M. (07 2016). "Stress Dose Steroids: Myths and Perioperative Medicine". Current Rheumatology Reports. 18 (7): 47. doi:10.1007/s11926-016-0595-7. ISSN 1534-6307. PMID 27351679.
{{cite journal}}: Check date values in:|date=(help) - ^ a b Pazderska, Agnieszka; Pearce, Simon HS (2017-6). "Adrenal insufficiency – recognition and management". Clinical Medicine. 17 (3): 258–262. doi:10.7861/clinmedicine.17-3-258. ISSN 1470-2118. PMC 6297573. PMID 28572228.
{{cite journal}}: Check date values in:|date=(help) - ^ Zelinka, Tomáš; Musil, Zdeněk; Dušková, Jaroslava; Burton, Deborah; Merino, Maria J; Milosevic, Dragana; Widimský, Jiří; Pacak, Karel (2011-10). "Metastatic pheochromocytoma: clinical, genetic, and histopathologic characteristics". European journal of clinical investigation. 41 (10): 1121–1128. doi:10.1111/j.1365-2362.2011.02518.x. ISSN 0014-2972. PMC 3170415. PMID 21692797.
{{cite journal}}: Check date values in:|date=(help) - ^ a b c Hamidi, Oksana; Young, William F.; Gruber, Lucinda; Smestad, John; Yan, Qi; Ponce, Oscar J.; Prokop, Larry; Murad, M. Hassan; Bancos, Irina (2017-11). "Outcomes of Patients with Metastatic Phaeochromocytoma and Paraganglioma: a Systematic Review and Meta-analysis". Clinical endocrinology. 87 (5): 440–450. doi:10.1111/cen.13434. ISSN 0300-0664. PMC 5854189. PMID 28746746.
{{cite journal}}: Check date values in:|date=(help) - ^ "Metastatic catecholamine-secreting paraganglioma (extra-adrenal pheochromocytoma)". The American Journal of Medicine. 61 (4): 523–532. 1976-10. doi:10.1016/0002-9343(76)90332-6. ISSN 0002-9343.
{{cite journal}}: Check date values in:|date=(help) - ^ Zelinka, Tomáš; Musil, Zdeněk; Dušková, Jaroslava; Burton, Deborah; Merino, Maria J.; Milosevic, Dragana; Widimský, Jiří; Pacak, Karel (2011-10). "Metastatic pheochromocytoma: does the size and age matter?". European Journal of Clinical Investigation. 41 (10): 1121–1128. doi:10.1111/j.1365-2362.2011.02518.x. ISSN 1365-2362. PMC 3170415. PMID 21692797.
{{cite journal}}: Check date values in:|date=(help) - ^ Engstrand, Jennie; Strömberg, Cecilia; Nilsson, Henrik; Freedman, Jacob; Jonas, Eduard (2019-12-26). "Synchronous and metachronous liver metastases in patients with colorectal cancer—towards a clinically relevant definition". World Journal of Surgical Oncology. 17 (1): 228. doi:10.1186/s12957-019-1771-9. ISSN 1477-7819. PMC 6933908. PMID 31878952.
{{cite journal}}: CS1 maint: PMC format (link) CS1 maint: unflagged free DOI (link) - ^ Corssmit, Eleonora P.M.; Snel, Marieke; Kapiteijn, Ellen (2020-01). "Malignant pheochromocytoma and paraganglioma". Current Opinion in Oncology. 32 (1): 20–26. doi:10.1097/cco.0000000000000589. ISSN 1040-8746.
{{cite journal}}: Check date values in:|date=(help) - ^ Roman-Gonzalez, Alejandro; Zhou, Shouhao; Ayala-Ramirez, Montserrat; Shen, Chan; Waguespack, Steven G.; Habra, Mouhammed A.; Karam, Jose A.; Perrier, Nancy; Wood, Christopher G.; Jimenez, Camilo (2018-07). "Impact of Surgical Resection of the Primary Tumor on Overall Survival in Patients With Metastatic Pheochromocytoma or Sympathetic Paraganglioma". Annals of Surgery. 268 (1): 172–178. doi:10.1097/sla.0000000000002195. ISSN 0003-4932.
{{cite journal}}: Check date values in:|date=(help) - ^ a b c Ellis, Ryan J; Patel, Dhaval; Prodanov, Tamara; Sadowski, Samira; Nilubol, Naris; Adams, Karen; Steinberg, Seth M; Pacak, Karel; Kebebew, Electron (2013-9). "Response after Surgical Resection of Metastatic Pheochromocytoma and Paraganglioma: Can Postoperative Biochemical Remission Be Predicted?". Journal of the American College of Surgeons. 217 (3): 489–496. doi:10.1016/j.jamcollsurg.2013.04.027. ISSN 1072-7515. PMC 3770940. PMID 23891076.
{{cite journal}}: Check date values in:|date=(help) - ^ Jimenez, Camilo; Rohren, Eric; Habra, Mouhammed Amir; Rich, Thereasa; Jimenez, Paola; Ayala-Ramirez, Montserrat; Baudin, Eric (2013-05-15). "Current and Future Treatments for Malignant Pheochromocytoma and Sympathetic Paraganglioma". Current Oncology Reports. 15 (4): 356–371. doi:10.1007/s11912-013-0320-x. ISSN 1523-3790.
- ^ Pappachan, Joseph M.; Raskauskiene, Diana; Sriraman, Rajagopalan; Edavalath, Mahamood; Hanna, Fahmy W. (2014-05-03). "Diagnosis and Management of Pheochromocytoma: A Practical Guide to Clinicians". Current Hypertension Reports. 16 (7). doi:10.1007/s11906-014-0442-z. ISSN 1522-6417.
- ^ BUHL, THORA; MORTENSEN, JANN; KJAER, ANDREAS (2002-03). "I-123 MIBG Imaging and Intraoperative Localization of Metastatic Pheochromocytoma". Clinical Nuclear Medicine. 27 (3): 183–185. doi:10.1097/00003072-200203000-00007. ISSN 0363-9762.
{{cite journal}}: Check date values in:|date=(help) - ^ De Filpo, G.; Maggi, M.; Mannelli, M.; Canu, L. (2020-06-29). "Management and outcome of metastatic pheochromocytomas/paragangliomas: an overview". Journal of Endocrinological Investigation. doi:10.1007/s40618-020-01344-z. ISSN 1720-8386.
- ^ Breen, William; Bancos, Irina; Young, William F.; Bible, Keith C.; Laack, Nadia N.; Foote, Robert L.; Hallemeier, Christopher L. (2017-11-22). "External beam radiation therapy for advanced/unresectable malignant paraganglioma and pheochromocytoma". Advances in Radiation Oncology. 3 (1): 25–29. doi:10.1016/j.adro.2017.11.002. ISSN 2452-1094. PMC 5856976. PMID 29556576.
- ^ Kohlenberg, Jacob; Welch, Brian; Hamidi, Oksana; Callstrom, Matthew; Morris, Jonathan; Sprung, Juraj; Bancos, Irina; Young, William (2019-02-07). "Efficacy and Safety of Ablative Therapy in the Treatment of Patients with Metastatic Pheochromocytoma and Paraganglioma". Cancers. 11 (2). doi:10.3390/cancers11020195. ISSN 2072-6694. PMC 6407137. PMID 30736463.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Niemeijer, N. D.; Alblas, G.; van Hulsteijn, L. T.; Dekkers, O. M.; Corssmit, E. P. M. (2014-11). "Chemotherapy with cyclophosphamide, vincristine and dacarbazine for malignant paraganglioma and pheochromocytoma: systematic review and meta-analysis". Clinical Endocrinology. 81 (5): 642–651. doi:10.1111/cen.12542. ISSN 1365-2265. PMID 25041164.
{{cite journal}}: Check date values in:|date=(help) - ^ Averbuch, S. D.; Steakley, C. S.; Young, R. C.; Gelmann, E. P.; Goldstein, D. S.; Stull, R.; Keiser, H. R. (1988-08-15). "Malignant pheochromocytoma: effective treatment with a combination of cyclophosphamide, vincristine, and dacarbazine". Annals of Internal Medicine. 109 (4): 267–273. doi:10.7326/0003-4819-109-4-267. ISSN 0003-4819. PMID 3395037.
- ^ a b Niemeijer, N. D.; Alblas, G.; van Hulsteijn, L. T.; Dekkers, O. M.; Corssmit, E. P. M. (2014-11). "Chemotherapy with cyclophosphamide, vincristine and dacarbazine for malignant paraganglioma and pheochromocytoma: systematic review and meta-analysis". Clinical Endocrinology. 81 (5): 642–651. doi:10.1111/cen.12542. ISSN 1365-2265. PMID 25041164.
{{cite journal}}: Check date values in:|date=(help) - ^ Huang, Hui; Abraham, Jame; Hung, Elizabeth; Averbuch, Steven; Merino, Maria; Steinberg, Seth M.; Pacak, Karel; Fojo, Tito (2008-10-15). "Treatment of malignant pheochromocytoma/paraganglioma with cyclophosphamide, vincristine, and dacarbazine: recommendation from a 22-year follow-up of 18 patients". Cancer. 113 (8): 2020–2028. doi:10.1002/cncr.23812. ISSN 0008-543X. PMID 18780317.
- ^ Nomura, Kaoru; Kimura, Hironari; Shimizu, Satoru; Kodama, Hitomi; Okamoto, Takahiro; Obara, Takao; Takano, Kazue (2009-08). "Survival of patients with metastatic malignant pheochromocytoma and efficacy of combined cyclophosphamide, vincristine, and dacarbazine chemotherapy". The Journal of Clinical Endocrinology and Metabolism. 94 (8): 2850–2856. doi:10.1210/jc.2008-2697. ISSN 1945-7197. PMID 19470630.
{{cite journal}}: Check date values in:|date=(help) - ^ Jawed, Irfan; Velarde, Margarita; Därr, Roland; Wolf, Katherine I.; Adams, Karen; Venkatesan, Aradhana M.; Balasubramaniam, Sanjeeve; Poruchynsky, Marianne S.; Reynolds, James C.; Pacak, Karel; Fojo, Tito (2018-7). "Continued tumor reduction of metastatic pheochromocytoma/paraganglioma harboring succinate dehydrogenase subunit B mutations with cyclical chemotherapy". Cellular and molecular neurobiology. 38 (5): 1099–1106. doi:10.1007/s10571-018-0579-4. ISSN 0272-4340. PMC 5976545. PMID 29623478.
{{cite journal}}: Check date values in:|date=(help) - ^ Tena, Isabel; Gupta, Garima; Tajahuerce, Marcos; Benavent, Marta; Cifrián, Manuel; Falcon, Alejandro; Fonfria, María; Del Olmo, Maribel; Reboll, Rosa; Conde, Antonio; Moreno, Francisca (2018). "Successful Second-Line Metronomic Temozolomide in Metastatic Paraganglioma: Case Reports and Review of the Literature". Clinical Medicine Insights. Oncology. 12: 1179554918763367. doi:10.1177/1179554918763367. ISSN 1179-5549. PMC 5922490. PMID 29720885.
- ^ Tong, Anli; Li, Ming; Cui, Yunying; Ma, Xiaosen; Wang, Huiping; Li, Yuxiu (2020). "Temozolomide Is a Potential Therapeutic Tool for Patients With Metastatic Pheochromocytoma/Paraganglioma-Case Report and Review of the Literature". Frontiers in Endocrinology. 11: 61. doi:10.3389/fendo.2020.00061. ISSN 1664-2392. PMC 7040234. PMID 32132978.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Hadoux, Julien; Favier, Judith; Scoazec, Jean-Yves; Leboulleux, Sophie; Al Ghuzlan, Abir; Caramella, Caroline; Déandreis, Désirée; Borget, Isabelle; Loriot, Céline; Chougnet, Cécile; Letouzé, Eric (2014-12-01). "SDHB mutations are associated with response to temozolomide in patients with metastatic pheochromocytoma or paraganglioma". International Journal of Cancer. 135 (11): 2711–2720. doi:10.1002/ijc.28913. ISSN 1097-0215. PMID 24752622.
- ^ a b Pryma, Daniel A.; Chin, Bennett B.; Noto, Richard B.; Dillon, Joseph S.; Perkins, Stephanie; Solnes, Lilja; Kostakoglu, Lale; Serafini, Aldo N.; Pampaloni, Miguel H.; Jensen, Jessica; Armor, Thomas (05 2019). "Efficacy and Safety of High-Specific-Activity 131I-MIBG Therapy in Patients with Advanced Pheochromocytoma or Paraganglioma". Journal of Nuclear Medicine: Official Publication, Society of Nuclear Medicine. 60 (5): 623–630. doi:10.2967/jnumed.118.217463. ISSN 1535-5667. PMC 6495236. PMID 30291194.
{{cite journal}}: Check date values in:|date=(help) - ^ a b Agrawal, Archi; Rangarajan, Venkatesh; Shah, Sneha; Puranik, Ameya; Purandare, Nilendu (2018-11). "MIBG (metaiodobenzylguanidine) theranostics in pediatric and adult malignancies". The British Journal of Radiology. 91 (1091). doi:10.1259/bjr.20180103. ISSN 0007-1285. PMC 6475939. PMID 30048149.
{{cite journal}}: Check date values in:|date=(help) - ^ Carrasquillo, Jorge A.; Pandit-Taskar, Neeta; Chen, Clara C. (2016-05). "I-131 Metaiodobenzylguanidine Therapy of Pheochromocytoma and Paraganglioma". Seminars in Nuclear Medicine. 46 (3): 203–214. doi:10.1053/j.semnuclmed.2016.01.011. ISSN 1558-4623. PMID 27067501.
{{cite journal}}: Check date values in:|date=(help) - ^ Kong, Grace; Grozinsky-Glasberg, Simona; Hofman, Michael S.; Callahan, Jason; Meirovitz, Amichay; Maimon, Ofra; Pattison, David A.; Gross, David J.; Hicks, Rodney J. (09 01, 2017). "Efficacy of Peptide Receptor Radionuclide Therapy for Functional Metastatic Paraganglioma and Pheochromocytoma". The Journal of Clinical Endocrinology and Metabolism. 102 (9): 3278–3287. doi:10.1210/jc.2017-00816. ISSN 1945-7197. PMID 28605448.
{{cite journal}}: Check date values in:|date=(help) - ^ Strosberg, Jonathan; Wolin, Edward; Chasen, Beth; Kulke, Matthew; Bushnell, David; Caplin, Martyn; Baum, Richard P.; Kunz, Pamela; Hobday, Timothy; Hendifar, Andrew; Oberg, Kjell (09 01, 2018). "Health-Related Quality of Life in Patients With Progressive Midgut Neuroendocrine Tumors Treated With 177Lu-Dotatate in the Phase III NETTER-1 Trial". Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 36 (25): 2578–2584. doi:10.1200/JCO.2018.78.5865. ISSN 1527-7755. PMC 6366953. PMID 29878866.
{{cite journal}}: Check date values in:|date=(help) - ^ Strosberg, Jonathan; El-Haddad, Ghassan; Wolin, Edward; Hendifar, Andrew; Yao, James; Chasen, Beth; Mittra, Erik; Kunz, Pamela L.; Kulke, Matthew H.; Jacene, Heather; Bushnell, David (01 12, 2017). "Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors". The New England Journal of Medicine. 376 (2): 125–135. doi:10.1056/NEJMoa1607427. ISSN 1533-4406. PMC 5895095. PMID 28076709.
{{cite journal}}: Check date values in:|date=(help) - ^ a b Vyakaranam, Achyut Ram; Crona, Joakim; Norlén, Olov; Granberg, Dan; Garske-Román, Ulrike; Sandström, Mattias; Fröss-Baron, Katarzyna; Thiis-Evensen, Espen; Hellman, Per; Sundin, Anders (2019-06-28). "Favorable Outcome in Patients with Pheochromocytoma and Paraganglioma Treated with 177Lu-DOTATATE". Cancers. 11 (7). doi:10.3390/cancers11070909. ISSN 2072-6694. PMC 6678507. PMID 31261748.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Satapathy, Swayamjeet; Mittal, Bhagwant Rai; Bhansali, Anil (12 2019). "'Peptide receptor radionuclide therapy in the management of advanced pheochromocytoma and paraganglioma: A systematic review and meta-analysis'". Clinical Endocrinology. 91 (6): 718–727. doi:10.1111/cen.14106. ISSN 1365-2265. PMID 31569282.
{{cite journal}}: Check date values in:|date=(help) - ^ Mak, Ingrid Y. F.; Hayes, Aimee R.; Khoo, Bernard; Grossman, Ashley (2019). "Peptide Receptor Radionuclide Therapy as a Novel Treatment for Metastatic and Invasive Phaeochromocytoma and Paraganglioma". Neuroendocrinology. 109 (4): 287–298. doi:10.1159/000499497. ISSN 1423-0194. PMID 30856620.
- ^ Wolf, Katherine I.; Jha, Abhishek; van Berkel, Anouk; Wild, Damian; Janssen, Ingo; Millo, Corina M.; Janssen, M. J. R.; Gonzales, Melissa K.; Timmers, Henri J. K. M.; Pacak, Karel (2019-06). "Eruption of Metastatic Paraganglioma After Successful Therapy with 177Lu/90Y-DOTATOC and 177Lu-DOTATATE". Nuclear Medicine and Molecular Imaging. 53 (3): 223–230. doi:10.1007/s13139-019-00579-w. ISSN 1869-3474. PMC 6554376. PMID 31231443.
{{cite journal}}: Check date values in:|date=(help) - ^ "NCI Dictionary of Cancer Terms - National Cancer Institute". www.cancer.gov. 2011-02-02. Retrieved 2020-08-18.
- ^ "Pheochromocytoma - National Cancer Institute". www.cancer.gov. 2020-02-12. Retrieved 2020-08-18.
- ^ Noshiro, Takao; Shimizu, Kazumasa; Watanabe, Toshiya; Akama, Hiroyoshi; Shibukawa, Satoru; Miura, Wakako; Ito, Sadayoshi; Miura, Yukio (2000-01-01). "Changes in clinical features and long-term prognosis in patients with pheochromocytoma". American Journal of Hypertension. 13 (1): 35–43. doi:10.1016/S0895-7061(99)00139-9. ISSN 0895-7061.
- ^ a b Hescot, Segolene; Curras-Freixes, Maria; Deutschbein, Timo; van Berkel, Anouk; Vezzosi, Delphine; Amar, Laurence; de la Fouchardière, Christelle; Valdes, Nuria; Riccardi, Fernando; Do Cao, Christine; Bertherat, Jerome (2019-06-01). "Prognosis of Malignant Pheochromocytoma and Paraganglioma (MAPP-Prono Study): A European Network for the Study of Adrenal Tumors Retrospective Study". The Journal of Clinical Endocrinology & Metabolism. 104 (6): 2367–2374. doi:10.1210/jc.2018-01968. ISSN 0021-972X.
- ^ Hamidi, Oksana (2019-06). "Metastatic pheochromocytoma and paraganglioma". Current Opinion in Endocrinology & Diabetes and Obesity. 26 (3): 146–154. doi:10.1097/med.0000000000000476. ISSN 1752-296X.
{{cite journal}}: Check date values in:|date=(help) - ^ Pacak, Karel; Eisenhofer, Graeme; Ahlman, Håkan; Bornstein, Stefan R; Gimenez-Roqueplo, Anne-Paule; Grossman, Ashley B; Kimura, Noriko; Mannelli, Massimo; McNicol, Anne Marie; Tischler, Arthur S (2007-02). "Pheochromocytoma: recommendations for clinical practice from the First International Symposium". Nature Clinical Practice Endocrinology & Metabolism. 3 (2): 92–102. doi:10.1038/ncpendmet0396. ISSN 1745-8366.
{{cite journal}}: Check date values in:|date=(help) - ^ a b Sugrue, Charles (1800). "A Case of Gastrodynia". Med Phys J. 4: 228–331.
- ^ Bausch, Birke; Tischler, Arthur S.; Schmid, Kurt W.; Leijon, Helena; Eng, Charis; Neumann, Hartmut P. H. (2017-07-01). "Max Schottelius: Pioneer in Pheochromocytoma". Journal of the Endocrine Society. 1 (7): 957–964. doi:10.1210/js.2017-00208. ISSN 2472-1972. PMC 5689150. PMID 29264546.
- ^ Fränkel, Felix (1886-02). "Ein Fall von doppelseitigem, völlig latent verlaufenen Nebennierentumor und gleichzeitiger Nephritis mit Veränderungen am Circulationsapparat und Retinitis". Archiv für Pathologische Anatomie und Physiologie und für Klinische Medicin. 103 (2): 244–263. doi:10.1007/bf01938677. ISSN 0945-6317.
{{cite journal}}: Check date values in:|date=(help) - ^ Kantorovich, Vitaly; Pacak, Karel (2010), "Pheochromocytoma and Paraganglioma", Progress in Brain Research, vol. 182, Elsevier, pp. 343–373, doi:10.1016/s0079-6123(10)82015-1, ISBN 978-0-444-53616-7, PMC 4714594, PMID 20541673, retrieved 2020-08-22
{{citation}}: CS1 maint: PMC format (link) - ^ Kiernan, Colleen M.; Solórzano, Carmen C. (2016-01). "Pheochromocytoma and Paraganglioma". Surgical Oncology Clinics of North America. 25 (1): 119–138. doi:10.1016/j.soc.2015.08.006. ISSN 1055-3207.
{{cite journal}}: Check date values in:|date=(help) - ^ a b Welbourn, R. B. (1987-07). "Early surgical history of phaeochromocytoma". The British Journal of Surgery. 74 (7): 594–596. doi:10.1002/bjs.1800740717. ISSN 0007-1323. PMID 3304519.
{{cite journal}}: Check date values in:|date=(help) - ^ a b MANGER, W. M (2006-08-01). "An Overview of Pheochromocytoma: History, Current Concepts, Vagaries, and Diagnostic Challenges". Annals of the New York Academy of Sciences. 1073 (1): 1–20. doi:10.1196/annals.1353.001. ISSN 0077-8923.
- ^ Jacob, Mathews; Macwana, Saurabh; Vivekanand, D (2015). "Anaesthetic management of a case of adrenal and extra-adrenal phaeochromocytoma for preoperative embolisation". Indian Journal of Anaesthesia. 59 (3): 196. doi:10.4103/0019-5049.153046. ISSN 0019-5049.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b Chen, Herbert; Sippel, Rebecca S.; Pacak, Karel (2010-8). "The NANETS Consensus Guideline for the Diagnosis and Management of Neuroendocrine Tumors: Pheochromocytoma, Paraganglioma & Medullary Thyroid Cancer". Pancreas. 39 (6): 775–783. doi:10.1097/MPA.0b013e3181ebb4f0. ISSN 0885-3177. PMC 3419007. PMID 20664475.
{{cite journal}}: Check date values in:|date=(help) - ^ Sutton, M. G.; Sheps, S. G.; Lie, J. T. (1981-06). "Prevalence of clinically unsuspected pheochromocytoma. Review of a 50-year autopsy series". Mayo Clinic Proceedings. 56 (6): 354–360. ISSN 0025-6196. PMID 6453259.
{{cite journal}}: Check date values in:|date=(help) - ^ Kim, Jung Hee; Moon, Hyemi; Noh, Junghyun; Lee, Juneyoung; Kim, Sin Gon (2020-3). "Epidemiology and Prognosis of Pheochromocytoma/Paraganglioma in Korea: A Nationwide Study Based on the National Health Insurance Service". Endocrinology and Metabolism. 35 (1): 157–164. doi:10.3803/EnM.2020.35.1.157. ISSN 2093-596X. PMC 7090309. PMID 32207276.
{{cite journal}}: Check date values in:|date=(help) - ^ Beard, C. M.; Sheps, S. G.; Kurland, L. T.; Carney, J. A.; Lie, J. T. (1983-12). "Occurrence of pheochromocytoma in Rochester, Minnesota, 1950 through 1979". Mayo Clinic Proceedings. 58 (12): 802–804. ISSN 0025-6196. PMID 6645626.
{{cite journal}}: Check date values in:|date=(help) - ^ Berends, Annika M.A.; Buitenwerf, Edward; de Krijger, Ronald R.; Veeger, Nic J.G.M.; van der Horst-Schrivers, Anouk N.A.; Links, Thera P.; Kerstens, Michiel N. (2018-05). "Incidence of pheochromocytoma and sympathetic paraganglioma in the Netherlands: A nationwide study and systematic review". European Journal of Internal Medicine. 51: 68–73. doi:10.1016/j.ejim.2018.01.015. ISSN 0953-6205.
{{cite journal}}: Check date values in:|date=(help) - ^ Ebbehoj, Andreas Ladefoged; Sondergaard, Esben; Trolle, Christian; Stochholm, Kirstine; Poulsen, Per Logstrup (2017-05-03). "The epidemiology of pheochromocytoma: increasing incidence and changing clinical presentation. A population-based retrospective study 1977-2015". Endocrine Abstracts. doi:10.1530/endoabs.49.oc1.4. ISSN 1479-6848.
- ^ a b c Aygun, Nurcihan; Uludag, Mehmet (2020-06-03). "Pheochromocytoma and Paraganglioma: From Epidemiology to Clinical Findings". The Medical Bulletin of Sisli Etfal Hospital. 54 (2): 159–168. doi:10.14744/SEMB.2020.18794. ISSN 1302-7123. PMC 7326683. PMID 32617052.
- ^ Antonio, Karren; Valdez, Ma Margarita Noreen; Mercado-Asis, Leilani; Taïeb, David; Pacak, Karel (2020-2). "Pheochromocytoma/paraganglioma: recent updates in genetics, biochemistry, immunohistochemistry, metabolomics, imaging and therapeutic options". Gland Surgery. 9 (1): 105–123. doi:10.21037/gs.2019.10.25. ISSN 2227-684X. PMC 7082276. PMID 32206603.
{{cite journal}}: Check date values in:|date=(help)CS1 maint: unflagged free DOI (link) - ^ "Current concepts of pheochromocytoma". International Journal of Surgery. 12 (5): 469–474. 2014-05-01. doi:10.1016/j.ijsu.2014.04.001. ISSN 1743-9191.
- ^ Boening, Andreas; Burger, Heiko (2018-1). ""If You Hear Hoof Beats, Think Horses, Not Zebras"". The Thoracic and Cardiovascular Surgeon Reports. 7 (1): e35. doi:10.1055/s-0038-1660808. ISSN 2194-7635. PMC 6033608. PMID 29984129.
{{cite journal}}: Check date values in:|date=(help) - ^ "Rare Disease Day 2021 - 28 Feb". Rare Disease Day - 28 Feb 2021. Retrieved 2020-08-26.
- ^ "Home". NORD (National Organization for Rare Disorders). Retrieved 2020-08-26.
- ^ "PRESSOR - Pheochromocytoma Research Support Organization". www.pressor.org. Retrieved 2020-08-26.
- ^ "The Alliance". Pheo Para Alliance. Retrieved 2020-08-26.
- ^ "Home". VHL Alliance. Retrieved 2020-08-26.
- ^ "Leading Funder Neuroendocrine Tumor Research". NETRF. Retrieved 2020-08-26.
- ^ "Rare Cancer : Rare Cancers". www.rare-cancer.org. Retrieved 2020-08-26.
- ^ Sanders, Lisa; M.D (2019-10-30). "Why Did the Young Mother Have Searing Head Pain and a Racing Heart?". The New York Times. ISSN 0362-4331. Retrieved 2020-08-26.
- ^ July 8; 2012 (2012-07-08). "Neuroendocrine Cancer Survivor Featured on Discovery Fit & Health TV Show". Carcinoid Cancer Foundation. Retrieved 2020-08-26.
{{cite web}}:|last2=has numeric name (help)CS1 maint: numeric names: authors list (link) - ^ "Acceptance". House Wiki. Retrieved 2020-08-26.
- ^ "Henry Burton". Grey's Anatomy Universe Wiki. Retrieved 2020-08-26.
- ^ Alliance, VHL Family. "VHL Family Alliance Applauds Grey's Anatomy for Featuring von Hippel-Lindau Disease". www.prnewswire.com. Retrieved 2020-08-26.
- ^ January 12, Kevin | Conditions |; 2011 (2011-01-12). "Mischaracterizations by the popular media of medical conditions". KevinMD.com. Retrieved 2020-08-26.
{{cite web}}:|last2=has numeric name (help)CS1 maint: numeric names: authors list (link) - ^ News, A. B. C. "'Grey's Anatomy' Features Rare Disease on Three-Episode Series". ABC News. Retrieved 2020-08-26.
{{cite web}}:|last=has generic name (help) - ^ "My Sacrificial Clam transcript". Scrubs Wiki. Retrieved 2020-08-26.