Redox (/ˈrɛdɒks/ RED-oks, /ˈriːdɒks/ REE-doks, reduction–oxidation[2] or oxidation–reduction[3]: 150 ) is a type of chemical reaction in which the oxidation states of a reactant change.[4] Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a decrease in the oxidation state.
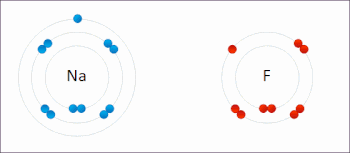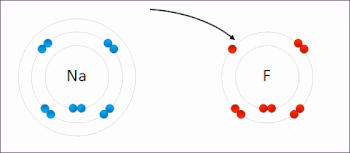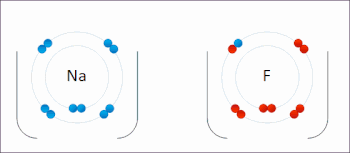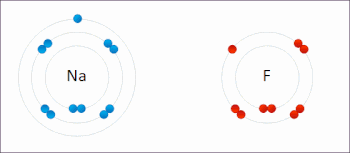
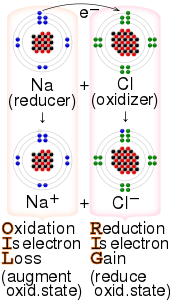
There are two classes of redox reactions:
- Electron-transfer – Only one (usually) electron flows from the atom, ion or molecule being oxidized to the atom, ion, or molecule that is reduced. This type of redox reaction is often discussed in terms of redox couples and electrode potentials.
- Atom transfer – An atom transfers from one substrate to another. For example, in the rusting of iron, the oxidation state of iron atoms increases as the iron converts to an oxide, and simultaneously the oxidation state of oxygen decreases as it accepts electrons released by the iron. Although oxidation reactions are commonly associated with the formation of oxides, other chemical species can serve the same function.[5] In hydrogenation, bonds like C=C are reduced by transfer of hydrogen atoms.
Terminology edit
"Redox" is a portmanteau of the words "reduction" and "oxidation". The term "redox" was first used in 1928.[6]
The processes of oxidation and reduction occur simultaneously and cannot occur independently.[5] In redox processes, the reductant transfers electrons to the oxidant. Thus, in the reaction, the reductant or reducing agent loses electrons and is oxidized, and the oxidant or oxidizing agent gains electrons and is reduced. The pair of an oxidizing and reducing agent that is involved in a particular reaction is called a redox pair. A redox couple is a reducing species and its corresponding oxidizing form,[7] e.g., Fe2+
/ Fe3+
.The oxidation alone and the reduction alone are each called a half-reaction because two half-reactions always occur together to form a whole reaction.[5]
Oxidants edit
Oxidation originally implied a reaction with oxygen to form an oxide. Later, the term was expanded to encompass substances that accomplished chemical reactions similar to those of oxygen. Ultimately, the meaning was generalized to include all processes involving the loss of electrons or the increase in the oxidation state of a chemical species.[8]: A49 Substances that have the ability to oxidize other substances (cause them to lose electrons) are said to be oxidative or oxidizing, and are known as oxidizing agents, oxidants, or oxidizers. The oxidant removes electrons from another substance, and is thus itself reduced.[8]: A50 Because it "accepts" electrons, the oxidizing agent is also called an electron acceptor. Oxidants are usually chemical substances with elements in high oxidation states[3]: 159 (e.g., N
2O
4, MnO−
4, CrO
3, Cr
2O2−
7, OsO
4), or else highly electronegative elements (e.g. O2, F2, Cl2, Br2, I2) that can gain extra electrons by oxidizing another substance.[3]: 909
Oxidizers are oxidants, but the term is mainly reserved for sources of oxygen, particularly in the context of explosions. Nitric acid is a strong oxidizer.[9]
Reductants edit
Substances that have the ability to reduce other substances (cause them to gain electrons) are said to be reductive or reducing and are known as reducing agents, reductants, or reducers. The reductant transfers electrons to another substance and is thus itself oxidized.[3]: 159 Because it donates electrons, the reducing agent is also called an electron donor. Electron donors can also form charge transfer complexes with electron acceptors. The word reduction originally referred to the loss in weight upon heating a metallic ore such as a metal oxide to extract the metal. In other words, ore was "reduced" to metal.[10] Antoine Lavoisier demonstrated that this loss of weight was due to the loss of oxygen as a gas. Later, scientists realized that the metal atom gains electrons in this process. The meaning of reduction then became generalized to include all processes involving a gain of electrons.[10] Reducing equivalent refers to chemical species which transfer the equivalent of one electron in redox reactions. The term is common in biochemistry.[11] A reducing equivalent can be an electron or a hydrogen atom as a hydride ion.[12]
Reductants in chemistry are very diverse. Electropositive elemental metals, such as lithium, sodium, magnesium, iron, zinc, and aluminium, are good reducing agents. These metals donate electrons relatively readily.[citation needed]
Hydride transfer reagents, such as NaBH4 and LiAlH4, reduce by atom transfer: they transfer the equivalent of hydride or H−. These reagents are widely used in the reduction of carbonyl compounds to alcohols.[13][14] A related method of reduction involves the use of hydrogen gas (H2) as sources of H atoms.[3]: 288
Electronation and deelectronation edit
The electrochemist John Bockris proposed the words electronation and deelectronation to describe reduction and oxidation processes, respectively, when they occur at electrodes.[15] These words are analogous to protonation and deprotonation.[16] They have not been widely adopted by chemists worldwide,[citation needed] although IUPAC has recognized the terms electronation[17] and de-electronation.[18]
Rates, mechanisms, and energies edit
This section needs expansion. You can help by adding to it. (April 2023) |
Redox reactions can occur slowly, as in the formation of rust, or rapidly, as in the case of burning fuel. Electron transfer reactions are generally fast, occurring within the time of mixing.[citation needed]
The mechanisms of atom-transfer reactions are highly variable because many kinds of atoms can be transferred. Such reactions can also be quite complex, involving many steps. The mechanisms of electron-transfer reactions occur by two distinct pathways, inner sphere electron transfer and outer sphere electron transfer.[citation needed]
Analysis of bond energies and ionization energies in water allow calculation of the thermodynamic aspects of redox reactions.[citation needed]
Standard electrode potentials (reduction potentials) edit
This section needs additional citations for verification. (December 2023) |
Each half-reaction has a standard electrode potential (Eo
cell), which is equal to the potential difference or voltage at equilibrium under standard conditions of an electrochemical cell in which the cathode reaction is the half-reaction considered, and the anode is a standard hydrogen electrode where hydrogen is oxidized:
- 1⁄2H2 → H+ + e−
The electrode potential of each half-reaction is also known as its reduction potential (Eo
red), or potential when the half-reaction takes place at a cathode. The reduction potential is a measure of the tendency of the oxidizing agent to be reduced. Its value is zero for H+ + e− → 1⁄2H2 by definition, positive for oxidizing agents stronger than H+ (e.g., +2.866 V for F2) and negative for oxidizing agents that are weaker than H+ (e.g., −0.763V for Zn2+).[8]: 873
For a redox reaction that takes place in a cell, the potential difference is:
- Eo
cell = Eo
cathode – Eo
anode
However, the potential of the reaction at the anode is sometimes expressed as an oxidation potential:
- Eo
ox = –Eo
red
The oxidation potential is a measure of the tendency of the reducing agent to be oxidized but does not represent the physical potential at an electrode. With this notation, the cell voltage equation is written with a plus sign
- Eo
cell = Eo
red(cathode) + Eo
ox(anode)
Examples of redox reactions edit
In the reaction between hydrogen and fluorine, hydrogen is being oxidized and fluorine is being reduced:
- H2 + F2 → 2 HF
This reaction is spontaneous and releases 542 kJ per 2 g of hydrogen because the H-F bond is much stronger than the F-F bond. This reaction can be analyzed as two half-reactions. The oxidation reaction converts hydrogen to protons:
The reduction reaction converts fluorine to the fluoride anion:
- F2 + 2 e− → 2 F−
The half reactions are combined so that the electrons cancel:
H
2→ 2 H+ + 2 e− F
2 + 2 e−→ 2 F−
H2 + F2 → 2 H+ + 2 F−
The protons and fluoride combine to form hydrogen fluoride in a non-redox reaction:
- 2 H+ + 2 F− → 2 HF
The overall reaction is:
- H2 + F2 → 2 HF
Metal displacement edit
In this type of reaction, a metal atom in a compound or solution is replaced by an atom of another metal. For example, copper is deposited when zinc metal is placed in a copper(II) sulfate solution:
- Zn (s) + CuSO4 (aq) → ZnSO4 (aq) + Cu (s)
In the above reaction, zinc metal displaces the copper(II) ion from copper sulfate solution and thus liberates free copper metal. The reaction is spontaneous and releases 213 kJ per 65 g of zinc.
The ionic equation for this reaction is:
- Zn + Cu2+ → Zn2+ + Cu
As two half-reactions, it is seen that the zinc is oxidized:
- Zn → Zn2+ + 2 e−
And the copper is reduced:
- Cu2+ + 2 e− → Cu
Other examples edit
- The reduction of nitrate to nitrogen in the presence of an acid (denitrification):
- 2 NO−3 + 10 e− + 12 H+ → N2 + 6 H2O
- The combustion of hydrocarbons, such as in an internal combustion engine, produces water, carbon dioxide, some partially oxidized forms such as carbon monoxide, and heat energy. Complete oxidation of materials containing carbon produces carbon dioxide.
- The stepwise oxidation of a hydrocarbon by oxygen, in organic chemistry, produces water and, successively: an alcohol, an aldehyde or a ketone, a carboxylic acid, and then a peroxide.
Corrosion and rusting edit
- The term corrosion refers to the electrochemical oxidation of metals in reaction with an oxidant such as oxygen. Rusting, the formation of iron oxides, is a well-known example of electrochemical corrosion: it forms as a result of the oxidation of iron metal. Common rust often refers to iron(III) oxide, formed in the following chemical reaction:
- 4 Fe + 3 O2 → 2 Fe2O3
- The oxidation of iron(II) to iron(III) by hydrogen peroxide in the presence of an acid:
- Fe2+ → Fe3+ + e−
- H2O2 + 2 e− → 2 OH−
- Here the overall equation involves adding the reduction equation to twice the oxidation equation, so that the electrons cancel:
- 2 Fe2+ + H2O2 + 2 H+ → 2 Fe3+ + 2 H2O
Disproportionation edit
A disproportionation reaction is one in which a single substance is both oxidized and reduced. For example, thiosulfate ion with sulfur in oxidation state +2 can react in the presence of acid to form elemental sulfur (oxidation state 0) and sulfur dioxide (oxidation state +4).
- S2O2−3 + 2 H+ → S + SO2 + H2O
Thus one sulfur atom is reduced from +2 to 0, while the other is oxidized from +2 to +4.[8]: 176
Redox reactions in industry edit
Cathodic protection is a technique used to control the corrosion of a metal surface by making it the cathode of an electrochemical cell. A simple method of protection connects protected metal to a more easily corroded "sacrificial anode" to act as the anode. The sacrificial metal, instead of the protected metal, then corrodes. A common application of cathodic protection is in galvanized steel, in which a sacrificial coating of zinc on steel parts protects them from rust.[citation needed]
Oxidation is used in a wide variety of industries such as in the production of cleaning products and oxidizing ammonia to produce nitric acid.[citation needed]
Redox reactions are the foundation of electrochemical cells, which can generate electrical energy or support electrosynthesis. Metal ores often contain metals in oxidized states such as oxides or sulfides, from which the pure metals are extracted by smelting at high temperature in the presence of a reducing agent. The process of electroplating uses redox reactions to coat objects with a thin layer of a material, as in chrome-plated automotive parts, silver plating cutlery, galvanization and gold-plated jewelry.[citation needed]
Redox reactions in biology edit
Bottom: dehydroascorbic acid (oxidized form of Vitamin C)
Many important biological processes involve redox reactions. Before some of these processes can begin iron must be assimilated from the environment.[19]
Cellular respiration, for instance, is the oxidation of glucose (C6H12O6) to CO2 and the reduction of oxygen to water. The summary equation for cell respiration is:
- C6H12O6 + 6 O2 → 6 CO2 + 6 H2O + Energy
The process of cell respiration also depends heavily on the reduction of NAD+ to NADH and the reverse reaction (the oxidation of NADH to NAD+). Photosynthesis and cellular respiration are complementary, but photosynthesis is not the reverse of the redox reaction in cell respiration:
- 6 CO2 + 6 H2O + light energy → C6H12O6 + 6 O2
Biological energy is frequently stored and released by means of redox reactions. Photosynthesis involves the reduction of carbon dioxide into sugars and the oxidation of water into molecular oxygen. The reverse reaction, respiration, oxidizes sugars to produce carbon dioxide and water. As intermediate steps, the reduced carbon compounds are used to reduce nicotinamide adenine dinucleotide (NAD+) to NADH, which then contributes to the creation of a proton gradient, which drives the synthesis of adenosine triphosphate (ATP) and is maintained by the reduction of oxygen. In animal cells, mitochondria perform similar functions.
Free radical reactions are redox reactions that occur as a part of homeostasis and killing microorganisms, where an electron detaches from a molecule and then reattaches almost instantaneously. Free radicals are a part of redox molecules and can become harmful to the human body if they do not reattach to the redox molecule or an antioxidant.
The term redox state is often used to describe the balance of GSH/GSSG, NAD+/NADH and NADP+/NADPH in a biological system such as a cell or organ. The redox state is reflected in the balance of several sets of metabolites (e.g., lactate and pyruvate, beta-hydroxybutyrate, and acetoacetate), whose interconversion is dependent on these ratios. Redox mechanisms also control some cellular processes. Redox proteins and their genes must be co-located for redox regulation according to the CoRR hypothesis for the function of DNA in mitochondria and chloroplasts.
Redox cycling edit
Wide varieties of aromatic compounds are enzymatically reduced to form free radicals that contain one more electron than their parent compounds. In general, the electron donor is any of a wide variety of flavoenzymes and their coenzymes. Once formed, these anion free radicals reduce molecular oxygen to superoxide and regenerate the unchanged parent compound. The net reaction is the oxidation of the flavoenzyme's coenzymes and the reduction of molecular oxygen to form superoxide. This catalytic behavior has been described as a futile cycle or redox cycling.
Redox reactions in geology edit
Minerals are generally oxidized derivatives of metals. Iron is mined as its magnetite (Fe3O4). Titanium is mined as its dioxide, usually in the form of rutile (TiO2). To obtain the corresponding metals, these oxides must be reduced, which is often achieved by heating these oxides with carbon or carbon monoxide as reducing agents. Blast furnaces are the reactors where iron oxides and coke (a form of carbon) are combined to produce molten iron.The main chemical reaction producing the molten iron is:[20]
- Fe2O3 + 3 CO → 2 Fe + 3 CO2
Redox reactions in soils edit
Electron transfer reactions are central to myriad processes and properties in soils, and redox potential, quantified as Eh (platinum electrode potential (voltage) relative to the standard hydrogen electrode) or pe (analogous to pH as -log electron activity), is a master variable, along with pH, that controls and is governed by chemical reactions and biological processes. Early theoretical research with applications to flooded soils and paddy rice production was seminal for subsequent work on thermodynamic aspects of redox and plant root growth in soils.[21] Later work built on this foundation, and expanded it for understanding redox reactions related to heavy metal oxidation state changes, pedogenesis and morphology, organic compound degradation and formation, free radical chemistry, wetland delineation, soil remediation, and various methodological approaches for characterizing the redox status of soils.[22][23]
Mnemonics edit
The key terms involved in redox can be confusing.[24][25] For example, a reagent that is oxidized loses electrons; however, that reagent is referred to as the reducing agent. Likewise, a reagent that is reduced gains electrons and is referred to as the oxidizing agent.[26] These mnemonics are commonly used by students to help memorise the terminology:[27]
- "OIL RIG" — oxidation is loss of electrons, reduction is gain of electrons[24][25][26][27]
- "LEO the lion says GER [grr]" — loss of electrons is oxidation, gain of electrons is reduction[24][25][26][27]
- "LEORA says GEROA" — the loss of electrons is called oxidation (reducing agent); the gain of electrons is called reduction (oxidizing agent).[26]
- "RED CAT" and "AN OX", or "AnOx RedCat" ("an ox-red cat") — reduction occurs at the cathode and the anode is for oxidation
- "RED CAT gains what AN OX loses" – reduction at the cathode gains (electrons) what anode oxidation loses (electrons)
- "PANIC" – Positive Anode and Negative is Cathode. This applies to electrolytic cells which release stored electricity, and can be recharged with electricity. PANIC does not apply to cells that can be recharged with redox materials. These galvanic or voltaic cells, such as fuel cells, produce electricity from internal redox reactions. Here, the positive electrode is the cathode and the negative is the anode.
See also edit
- Anaerobic respiration
- Bessemer process
- Bioremediation
- Calvin cycle
- Chemical equation
- Chemical looping combustion
- Citric acid cycle
- Electrochemical series
- Electrochemistry
- Electrolysis
- Electron equivalent
- Electron transport chain
- Electrosynthesis
- Galvanic cell
- Hydrogenation
- Membrane potential
- Microbial fuel cell
- Murburn concept
- Nucleophilic abstraction
- Organic redox reaction
- Oxidative addition and reductive elimination
- Oxidative phosphorylation
- Partial oxidation
- Pro-oxidant
- Redox gradient
- Redox potential
- Reducing agent
- Reducing atmosphere
- Reduction potential
- Thermic reaction
- Transmetalation
- Sulfur cycle
References edit
- ^ "Metals". Bitesize. BBC. Archived from the original on November 3, 2022.
- ^ "redox – definition of redox in English | Oxford Dictionaries". Oxford Dictionaries | English. Archived from the original on October 1, 2017. Retrieved May 15, 2017.
- ^ a b c d e Petrucci, Ralph H.; Harwood, William S.; Herring, F. Geoffrey (2002). General Chemistry (8th ed.). Prentice-Hall. ISBN 0-13-014329-4.
- ^ "Redox Reactions". wiley.com. Archived from the original on May 30, 2012. Retrieved May 9, 2012.
- ^ a b c Haustein, Catherine Hinga (2014). "Oxidation-reduction reaction". In K. Lee Lerner; Brenda Wilmoth Lerner (eds.). The Gale Encyclopedia of Science (5th ed.). Farmington Hills, MI: Gale Group.
- ^ Harper, Douglas. "redox". Online Etymology Dictionary.
- ^ Pingarrón, José M.; Labuda, Ján; Barek, Jiří; Brett, Christopher M. A.; Camões, Maria Filomena; Fojta, Miroslav; Hibbert, D. Brynn (2020). "Terminology of electrochemical methods of analysis (IUPAC Recommendations 2019)". Pure and Applied Chemistry. 92 (4): 641–694. doi:10.1515/pac-2018-0109.
- ^ a b c d Petrucci, Ralph H.; Harwood, William S.; Herring, F. Geoffrey (2017). General Chemistry: Principles and Modern applications (11th ed.). Toronto: Pearson. ISBN 978-0-13-293128-1.
- ^ "Nitric Acid Fact Sheet" (PDF). Department of Environmental Safety, Sustainability & Risk. University of Maryland. Retrieved February 12, 2024.
- ^ a b Whitten, Kenneth W.; Gailey, Kenneth D.; Davis, Raymond E. (1992). General Chemistry (4th ed.). Saunders College Publishin. p. 147. ISBN 0-03-072373-6.
- ^ Jain JL (2004). Fundamentals of Biochemistry. S. Chand. ISBN 81-219-2453-7.
- ^ Lehninger AL, Nelson DL, Cox MM (January 1, 2017). Lehninger Principles of Biochemistry (Seventh ed.). New York, NY. ISBN 9781464126116. OCLC 986827885.
{{cite book}}: CS1 maint: location missing publisher (link) - ^ Hudlický, Miloš (1996). Reductions in Organic Chemistry. Washington, D.C.: American Chemical Society. p. 429. ISBN 978-0-8412-3344-7.
- ^ Hudlický, Miloš (1990). Oxidations in Organic Chemistry. Washington, D.C.: American Chemical Society. pp. 456. ISBN 978-0-8412-1780-5.
- ^ Bockris, John O'M.; Reddy, Amulya K. N. (1970). Modern Electrochemistry. Plenum Press. pp. 352–3.
- ^ Bockris, John O'M.; Reddy, Amulya K.N. (2013) [1970]. Modern Electrochemistry. Vol. 1. Springer Science & Business Media. p. 494. ISBN 9781461574675. Retrieved March 29, 2020.
The homogeneous proton-transfer reactions described are similar to homogeneous electron-transfer reactions in that the overall electron-transfer reaction can be decomposed into one electronation reaction and one deelectronation reaction.
- ^ IUPAC. Compendium of Chemical Terminology, 2nd ed. (the "Gold Book"). Compiled by A. D. McNaught and A. Wilkinson. Blackwell Scientific Publications, Oxford (1997). Online version (2019-) created by S. J. Chalk. ISBN 0-9678550-9-8. https://goldbook.iupac.org/terms/view/R05222
- ^ IUPAC. Compendium of Chemical Terminology, 2nd ed. (the "Gold Book"). Compiled by A. D. McNaught and A. Wilkinson. Blackwell Scientific Publications, Oxford (1997). Online version (2019-) created by S. J. Chalk. ISBN 0-9678550-9-8. https://goldbook.iupac.org/terms/view/O04362
- ^ "Titles of Volumes 1–44 in the Metal Ions in Biological Systems Series". Metals, Microbes, and Minerals - the Biogeochemical Side of Life. De Gruyter. 2021. pp. xxiii–xxiv. doi:10.1515/9783110589771-005. ISBN 9783110588903. S2CID 242013948.
- ^ Oeters, Franz; Ottow, Manfred; Meiler, Heinrich; Lüngen, Hans Bodo; Koltermann, Manfred; Buhr, Andreas; Yagi, Jun-Ichiro; Formanek, Lothar; Rose (2006). "Iron". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a14_461.pub2. ISBN 978-3527306732.
- ^ Ponnamperuma, Felix Nelson (1992). "The chemistry of submerged soils". Advances in Agronomy. 24: 29–96. doi:10.1016/S0065-2113(08)60633-1. ISBN 9780120007240. Retrieved September 10, 2023.
- ^ Bartlett, Richmond J.; James, Bruce R. (1991). "Redox chemistry of soils". Advances in Agronomy. 39: 151–208.
- ^ James, Bruce R.; Brose, Dominic A. (2012). "Oxidation-reduction phenomena". In Huang, Pan Ming; Li, Yuncong; Sumner, Malcolm E. (eds.). Handbook of soil sciences: properties and processes (second ed.). Boca Raton, Florida: CRC Press. pp. 14-1 -- 14-24. ISBN 978-1-4398-0305-9.
- ^ a b c Robertson, William (2010). More Chemistry Basics. National Science Teachers Association. p. 82. ISBN 978-1-936137-74-9.
- ^ a b c Phillips, John; Strozak, Victor; Wistrom, Cheryl (2000). Chemistry: Concepts and Applications. Glencoe McGraw-Hill. p. 558. ISBN 978-0-02-828210-7.
- ^ a b c d Rodgers, Glen (2012). Descriptive Inorganic, Coordination, and Solid-State Chemistry. Brooks/Cole, Cengage Learning. p. 330. ISBN 978-0-8400-6846-0.
- ^ a b c Zumdahl, Steven; Zumdahl, Susan (2009). Chemistry. Houghton Mifflin. p. 160. ISBN 978-0-547-05405-6.
Further reading edit
- Schüring, J.; Schulz, H. D.; Fischer, W. R.; Böttcher, J.; Duijnisveld, W. H., eds. (1999). Redox: Fundamentals, Processes and Applications. Heidelberg: Springer-Verlag. p. 246. hdl:10013/epic.31694.d001. ISBN 978-3-540-66528-1.
- Tratnyek, Paul G.; Grundl, Timothy J.; Haderlein, Stefan B., eds. (2011). Aquatic Redox Chemistry. ACS Symposium Series. Vol. 1071. doi:10.1021/bk-2011-1071. ISBN 978-0-8412-2652-4.