The phenylpropanoids are a diverse family of organic compounds that are synthesized by plants from the amino acid phenylalanine. Their name is derived from the six-carbon, aromatic phenyl group and the three-carbon propene tail of cinnamic acid, which is synthesized from phenylalanine in the first step of phenylpropanoid biosynthesis. Phenylpropanoids are found throughout the plant kingdom, where they serve as essential components of a number of structural polymers, provide protection from ultraviolet light, defend against herbivores and pathogens, and mediate plant-pollinator interactions as floral pigments and scent compounds.
Cinnamic acid is the precursor of all phenylpropanoids, and is synthesized via the deamination of phenylalanine. Reduction of the carboxylic acid group of cinnamic acid gives rise to cinnamaldehyde and cinnamyl alcohol. Other phenylpropanoids can be grouped into various subclasses based on their synthesis and structure.
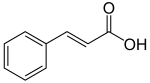
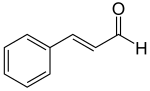
Hydroxycinnamic acids
editHydroxycinnamic acids consist of a cinnamic acid moeity to which various hydroxyl, methoxyl, or other groups have been added. Hydroxycinnamic acids commonly found in nature include p-coumaric acid (4-hydroxycinnamic acid), caffeic acid (3,4-dihydroxycinnamic acid), ferulic acid (3-methoxy 4-hydroxycinnamic acid), and sinapic acid (3,5-dimethoxy 4-hydroxycinnamic acid). Although the hydroxycinnamic acids were long thought to be derived from direct hydroxylation and methylation of cinnamic acid, more recent evidence has revealed a more complicated set of biochemical reactions is responsible for their synthesis.
Hydroxycinnamaldehydes and hydroxycinnamyl alcohols (monolignols)
editHydroxycinnamaldehydes and hydroxycinnamyl alcohols are similar in structure to hydroxycinnamic acids, but contain an aldehyde or alcohol functional group in place of the propene tail carboxylic acid group. The hydroxycinnamyl alcohols p-coumaryl alcohol, coniferyl alcohol, and sinapyl alcohol are often referred to as monolignols, due to their role as monomers for the production of lignans (monolignol dimers) and lignin (a complex heteropolymer). Again, the long accepted biosynthetic pathway for the production of hydroxycinnamaldehydes and hydroxycinnamyl alcohols (direct reduction of the corresponding hydroxycinnamic acid) has been shown to be more complicated than previously thought.
Phenylpropenes
editPhenylpropenes consist of a substituted phenyl ring and a propene tail (lacking any carboxylic acid, aldehyde, or alcohol groups). The double bond of the propene tail can either be between the first and second carbon of the tail (e.g. anol or isoeugenol), or between the second and third carbon (e.g. chavicol or eugenol). These compounds are the primary constituents of various essential oils.
Hydroxycinnamate esters
editHydroxycinnamate esters are derived from the esterification of hydroxcinnamic acids to various acyl acceptors. Examples of hydroxycinnamate esters include ethyl cinnamate (a floral scent compound), sinapoylmalate (a UV protectant), and chlorogenic acid (caffeoylquinate, an anti-herbivory agent).
Flavonoids and stilbenoids
editFlavonoids and stilbenoids are derived from the addition of three molecules of malonyl-CoA to a hydroxycinnamic acid (often p-coumaric acid). Cyclization of these malonyl groups yields three rings in the case of flavonoids, and two rings in the case of stilbenoids. Flavonoids are further divided into subgroups including anthocyanins, flavonols, and condensed tannins.
Coumarins
editCoumarins result from the formation of a second aromatic ring between the carboxylic acid group of the propene tail of a hydroxycinnamic acid and a hydroxyl group on the phenyl ring. Examples include coumarin (from which the name of the group is derived), umbelliferone, and scopoletin.
References
edit- K Hahlbrock, D Scheel (1989). "Physiology and Molecular Biology of Phenylpropanoid Metabolism". Annual Review of Plant Physiology and Plant Molecular Biology. 40: 347–69. doi:10.1146/annurev.pp.40.060189.002023.