 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Genticyn, Garamycin, Magenta, Merigenta and others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682275 |
| Pregnancy category |
|
| Routes of administration | IV, eye drop, IM, topical |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | limited oral bioavailability |
| Protein binding | 0-10% |
| Elimination half-life | 2 hrs |
| Excretion | renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
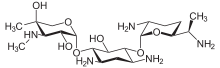
| Formula | C21H43N5O7 |
| Molar mass | 477.596 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Gentamicin, sold under the brandname Garamycin, Cidomycin, and Septopal, is an antibiotic used to treat many types of bacterial infections.[1] Gentamicin is a type of aminoglycoside.It kills bacteria by stopping protein production in the bacteria.[1] This may include bone infections, endocarditis, pelvic inflammatory disease, meningitis, pneumonia, urinary tract infections, and sepsis among others. It is not effective for gonorrhea or chlamydia infections. It can be given intravenously, by injection into a muscle, or topically.[1] Topical formulations may be used in burns or for infections of the outside of the eye.[2] In the developed world it is often only used for two days until bacterial cultures determine what antibiotics the infection is sensitive to.[3] The dose required should be monitored by blood testing.[1]
Gentamicin can cause inner ear problems and kidney problems.[1] The inner ear problems can include problems with balance and problems with hearing. These problems may be permanent.
Gentamicin was discovered in 1963.[4] It is made from the bacteria Micromonospora purpurea.[1] Gentamicin is on the World Health Organization's List of Essential Medicines, the most important medication needed in a basic health system.[5] It is available as a generic medication.[6] It wholesale cost is between 0.05 and 0.58 USD per day.[7]
Medical uses edit
Active against a wide range of bacterial infections, mostly Gram-negative bacteria including Pseudomonas, Proteus, Escherichia coli, Klebsiella pneumoniae, Enterobacter aerogenes, Serratia, and the Gram-positive Staphylococcus.[8] Gentamicin is used in the treatment of respiratory tract infections, urinary tract infections, blood, bone and soft tissues infections of these susceptible bacteria.[9]
Gentamicin is not used for Neisseria gonorrhoeae, Neisseria meningitidis or Legionella pneumophila bacterial infections (because of the risk of the person going into shock from lipid A endotoxin found in certain Gram-negative organisms). Gentamicin is also useful against Yersinia pestis, its relatives, and Francisella tularensis (the organism responsible for tularemia seen often in hunters and/or trappers).[10]
Some Enterobacteriaceae, Pseudomonas spp., enterococci, Staphylococcus aureus and other staphylococci are resistant to gentamicin sulfate, to varying degrees.[11]
Side effects edit
Side effects of gentamicin can range from less severe reactions such as nausea and vomiting to more severe reactions such as low blood counts, allergic responses, neuromuscular problems, neuropathy, kidney and ear disorders.[8] Nephrotoxicity and ototoxicity are thought to be dose related with higher doses causing greater chance of toxicity. [9] These two toxicities may have delayed presentation, sometimes not appearing until after completing treatment. [8]
Nephrotoxicity edit
Nephrotoxicity is a problem in 10-25% of patients who receive aminoglycosides, and gentamicin is one of the most nephrotoxic of the class.[12] Often times acute nephrotoxicity is reversible, but it may be fatal.[8] The risk of nephrotoxicity can be affected by the dose, frequency, duration of therapy, and concurrent use of certain medications such as NSAIDs, diuretics, cisplatin, cyclosporin, cephalosporin, amphotericin, iodide contrast media, and vancomycin .[12]
Patient factors that increase risk of nephrotoxicity include: [12]
- increased age
- reduced renal function
- pregnancy
- hypothyroidism
- hepatic dysfunction
- volume depletion
- metabolic acidosis
- sodium depletion
Gentamicin, like other aminoglycosides, causes nephrotoxicity by inhibiting protein synthesis in renal cells. This mechanism specifically causes necrosis of cells in the proximal tubule, resulting in acute tubular necrosis which can lead to acute renal failure.[13] Kidney dysfunction is monitored by measuring creatinine in the blood, electrolyte levels, and concentrations of other chemical in the blood.[12]
Ototoxicity edit
11% of the population who receives aminoglycosides experience ototoxicity.[14] The common symptoms of ototoxicity are: tinnitus, hearing loss, vertigo, ataxia, dizziness.[15] Chronic use of gentamicin can affect two area of the ears. First, damage of the inner ear hair cells can result in irreversible hearing loss. Second, damage to inner ear vestibular apparatus can lead to balance problems.[16] To reduce the risk of ototoxicity it is recommended to stay hydrated.[15]
Patient factors that increase risk of ototoxicity include:[15][17]
- Uremic
- Renal dysfunction
- Liver dysfunction
- Higher doses
- Long courses of therapy
- Concomitant use of potent diuretics (eg. furosemide)
Mechanism of action edit
Gentamicin is a bactericidal antibiotic that works by irreversibly binding the 30S subunit of the bacterial ribosome, interrupting protein synthesis. This mechanism of action is similar to other aminoglycosides.[18] Gentamicin is a bactericidal antibiotic.
Components edit
Gentamicin is composed of a number of related gentamicin components and fractions which have varying degrees of antimicrobial potency.[19] The main components of gentamicin include members of the gentamicin C complex: gentamicin C1, gentamicin C1a, and gentamicin C2 which compose approximately 80% of gentamicin and have been found to have the highest antibacterial activity. Gentamicin A, B, X, and a few others make up the remaining 20% of gentamicin and have lower antibiotic activity than the gentamicin C complex.[20] The exact composition of a given sample or lot of gentamicin is not well defined, and the level of gentamicin C components or other components in gentamicin may differ from lot-to-lot depending on the gentamicin manufacturer or manufacturing process. Because of this lot-to-lot variability, it can be difficult to study various properties of gentamicin including pharmacokinetics and microorganism susceptibility if there is an unknown combination of chemically related but different compounds.[21]
Contraindications edit
Gentamicin should not be used if a patient has a history of hypersensitivity or other serious toxic reaction to gentamicin or any other aminoglycosides.[9]
Special Populations edit
Pregnancy and Breastfeeding edit
Gentamicin is not recommended in pregnancy unless the benefits outweigh the risks for the mother e.g. life threatening situations. Gentamicin can cross the placenta and several reports of irreversible bilateral congenital deafness in children have been seen. Intramuscular injection of gentamicin in mothers can cause muscle weakness in the newborn.[9]
The safety and efficacy for gentamicin in nursing mothers has not been established. Detectable gentamicin levels are found in human breast milk and in nursing infants.[9]
Geriatrics edit
Renal function monitoring should be assessed prior to initiation and during therapy in elderly due to age-related decline in glomerular filtration rate. Excretion of gentamicin may take longer in this population, which can prolong gentamicin levels in the body. Use cautiously in persons with renal, auditory, vestibular, or neuromuscular dysfunction.[8]
Pediatrics edit
Gentamicin may not be appropriate for use in children, including newborns and infants. Studies have shown higher serum levels and a longer half-life in this population. Assess renal function periodically during therapy. Hypocalcemia, hypokalemia, and muscle weakness have been reported after gentamicin injection.[8]
History edit
Gentamicin is produced by the fermentation of Micromonospora purpurea. It was discovered in 1963 by Weinstein, Wagman et al. at Schering Corporation in Bloomfield, N.J. working with source material (soil samples) provided by Rico Woyciesjes.[22] Subsequently it was purified and the structures of its three components determined by Cooper, et al., also at the Schering Corporation. It was initially used as a topical treatment for burns at the Atlanta and San Antonio burn units and was introduced into IV usage in 1971. It remains a mainstay for use in sepsis.
It is synthesized by Micromonospora, a genus of Gram-positive bacteria widely present in the environment (water and soil). To highlight their specific biological origins, gentamicin and other related antibiotics produced by this genus (verdamicin, mutamicin, sisomicin, netilmicin, retymicin) generally have their spellings ending in ~micin and not in ~mycin.
Research edit
Gentamicin is also used in molecular biology research as an antibacterial agent in tissue and cell culture, to prevent contamination of sterile cultures. Gentamicin is one of the few heat-stable antibiotics that remain active even after autoclaving, which makes it particularly useful in the preparation of some microbiological growth media.[citation needed]
References edit
- ^ a b c d e f "Gentamicin Sulfate". The American Society of Health-System Pharmacists. Retrieved Aug 15, 2015.
- ^ Bartlett, Jimmy (2013). Clinical Ocular Pharmacology (s ed.). Elsevier. p. 214. ISBN 9781483193915.
- ^ Moulds, Robert and Jeyasingham, Melanie (October 2010). "Gentamicin: a great way to start". Australian Prescriber. 33 (5): 134–135. doi:10.18773/austprescr.2010.062.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Pucci, edited by Thomas Dougherty, Michael J.; Weinstein, Marvin J. (2011). Handbook of antibiotic discovery and development (2012 ed.). New York: Springer. p. 238. ISBN 9781461413998.
{{cite book}}:|first1=has generic name (help)CS1 maint: multiple names: authors list (link) - ^ "WHO Model List of EssentialMedicines" (PDF). World Health Organization. October 2013. Retrieved 22 April 2014.
- ^ Burchum, Jacqueline (2014). Lehne's Pharmacology for Nursing Care. Elsevier Health Sciences. p. 1051. ISBN 9780323340267.
- ^ "Gentamicin Sulfate". International Drug Price Indicator Guide. Retrieved 15 August 2015.
- ^ a b c d e f "Gentamicin" (PDF). Baxter Corporation. Retrieved 2 November 2015.
- ^ a b c d e "Product Monograph" (PDF). Sandoz Canada Inc. Retrieved 2 November 2015.
- ^ Goljan, Edward F. (2011). Rapid Review Pathology (3rd ed.). Philadelphia, PA: Elsevier. p. 241. ISBN 978-0-323-08438-3.
- ^ "Gentamicin spectrum of bacterial susceptibility and Resistance" (PDF). Retrieved 15 May 2012.
- ^ a b c d Lopez-Novoa, Jose (2011). "New insights into the mechanism of aminoglycoside nephrotoxicity: an integrative point of view". Kidney International. 79 (1): 33–45. doi:10.1038/ki.2010.337. PMID 20861826. Retrieved September 30, 2014.
- ^ Sundin, D.P.; Sandoval, R.; Molitoris, B.A. (2001). "Gentamicin Inhibits Renal Protein and Phospholipid Metabolism in Rats: Implications Involving Intracellular Trafficking". J Am Soc Nephrol. 12 (1): 114–123. PMID 11134257.
- ^ East, J E; Foweraker, J E; Murgatroyd, F D (2005-05-01). "Gentamicin induced ototoxicity during treatment of enterococcal endocarditis: resolution with substitution by netilmicin". Heart. 91 (5): e32. doi:10.1136/hrt.2003.028308. ISSN 1355-6037. PMC 1768868. PMID 15831617.
- ^ a b c "Gentamicin" (PDF). Baxter Corporation. Retrieved 2 November 2015.
- ^ Selimoglu, Erol (2007-01-01). "Aminoglycoside-induced ototoxicity". Current Pharmaceutical Design. 13 (1): 119–126. doi:10.2174/138161207779313731. ISSN 1873-4286. PMID 17266591.
- ^ "Product Monograph" (PDF). Sandoz Canada Inc. Retrieved 2 November 2015.
- ^ http://www.drugbank.ca/drugs/DB00798.
{{cite web}}: Missing or empty|title=(help) - ^ Weinstein, Marvin J. (1967). "Biological Activity of the Antibiotic Components of the Gentamicin Complex". Journal of Bacteriology. 94 (3): 789–790. doi:10.1128/JB.94.3.789-790.1967. PMC 251956. PMID 4962848.
- ^ Vydrin, A. F. (2003). "Component Composition of Gentamicin Sulfate Preparations". Pharmaceutical Chemistry Journal. 37 (8): 448–449. doi:10.1023/A:1027372416983. S2CID 43731658.
- ^ Isoherranen, Nina; Eran, Lavy (2000). "Pharmacokinetics of Gentamicin C1, C1a, and C2 in Beagles after a Single Intravenous Dose". Antimicrobial Agents and Chemotherapy. 44 (6): 1443–1447. doi:10.1128/AAC.44.6.1443-1447.2000. PMC 89894. PMID 10817690.
- ^ Weinstein, Marvin; Wagman (1963). "Gentamicin, A New Antimicrobial Complex from Micromonospora". J Med Chem. 6: 463–464. doi:10.1021/jm00340a034. PMID 14184912.
Category:Aminoglycoside antibiotics Category:World Health Organization essential medicines Category:Toxicology Category:Otologicals