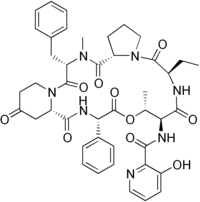
Virginiamycin S1 is a macrolide antibiotic in the group of antibiotics known as streptogramin B.[1]
| |
| Names | |
|---|---|
| Systematic IUPAC name
N-[(6R,9S,10R,13S,15aS,22S,24aS)-22-Benzyl-6-ethyl-10,23-dimethyl-5,8,12,15,17,21,24-heptaoxo-13-phenyldocosahydro-12H-pyrido[2,1-f]pyrrolo[2,1-l] [1,4,7,10,13,16]oxapentaazacyclononadecin-9-yl]-3-hydroxypyridine-2-carboxamide | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.041.314 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C43H49N7O10 | |
| Molar mass | 823.904 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
References
edit- ^ Kingston, David G. I.; Molinero, Anthony A.; Purvis, Michael B.; Reed, Josephine W.; LeFevre, Joseph W. (1989). "Studies in the biosynthesis of antibiotics of the virginiamycin family". Revista Latinoamericana de Química. 20 (3–4): 128–132.
{{cite journal}}: CS1 maint: multiple names: authors list (link)