Blood cell formation, also known as haematopoiesis, is the process of production of blood cellular components. In a healthy adult, approximately 500 billion blood cells[1] are produced per day to maintain a constant internal environment for the body to function normally.
Haematopoietic Stem Cells (HSCs)
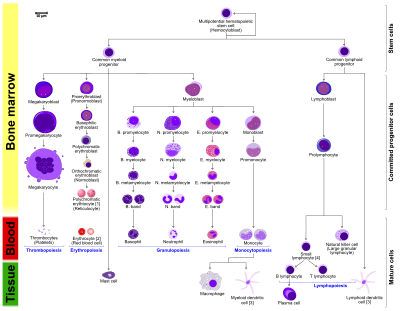
editHaematopoietic stem cells (HSCs) are stem cells which have the unique ability to give rise to other types of blood cells through haematopoiesis. HSCs are self-renewing cells. Some of them stay in the G0 phase (quiescent) of the cell division until being induced by cytokines to initiate G1 phase of the cell division[2]. This prevents the depletion of HSCs. This kind of division is known as asymmetric division[3]. One HSC is capable to produce the entire haematopoietic system[2]. Daughter cells of parent HSCs, common myeloid progenitors (CMPs) and common lymphoid progenitors (CLPs) can then differentiate into lineage-restricted cells. Daughter cells of MSCs lost the self-renewing property[2]. All blood cells derived from CMPs and CLPs can be divided into three lineages[4]. CMPs can form erythrocytes, thrombocytes, macrophages and granulocytes. CLPs can from lymphocytes such as natural killer cells, T-cells and B-cells.
Irregulation or destruction of HSCs may lead to many blood disorders such as aplastic anaemia, leukaemia and myelodysplastic syndromes[5].
Location
editBlood cell formation occurs in different regions during different stages of development. During the embryonic development, blood cell formation mainly occurs in the yolk sac, as known as blood islands, where blood cells aggregate. As development progresses, major sites of blood formation shift to the lymph nodes, liver and spleen. After bone marrow is completely developed, most of the blood cell formation processes take place in bone marrow eventually for the entire organism.[6] However, some processes like activation, lymphoid cell proliferation and maturation take place in the lymph nodes, thymus, and spleen. In childhood development stage, only marrow of long bones like the tibia and femur are responsible for blood cell formation, also known as haematopoiesis. In the adult development stage, haematopoiesis only takes place in the marrow of sternum, cranium pelvis, and vertebrae.[7] HSCs can only be found in marrow of bones like femur, sternum and pelvis or in blood such as peripheral blood or umbilical cord blood.
Common Myeloid Progenitors (CMPs)
editThrombocytes
editThrombocytes, also known as platelets, are responsible for handling wounds of inflammation and bleeding from blood vessels by forming a blood clot through clumping[8]. They are fragments of cytoplasm that are derived from megakaryocytes of the bone marrow[9], through the process of megakaryocytopoiesis and thrombopoiesis[10]. Mature thrombocytes have a biconvex discoid shape and no nucleus[11][12].
Thrombopoiesis starts with multipotent HSCs. They differentiate into multipotent progenitors, followed by CMPs, then megakaryoblasts, then megakaryocyte-erythroid progenitors, then megakaryocytes and finally thrombocytes[2]. Thrombopoiesis is regulated by thrombopoietin, a glycoprotein hormone produced by the liver and kidney. Thrombopoietin stimulates production and differentiation of megakaryocytes[13]. A megakaryocyte can differentiate into several thousand platelets[2].
Thrombopoiesis is highly regulated. Uncontrolled regulation of thrombopoiesis may lead to thrombocytopenia, an abnormally low amount of thrombocytes[14] and thrombocythemia, an abnormally elevated amount of thrombocytes. Causes of thrombocytopenia include insufficient production of thrombopoietin and folate acid, vitamin B12 insufficiency. Thrombocytopenia may result in continuous bleeding. Causes of thrombocythemia include acute inflammation and malignancy, which results in elevated risks of thrombosis and thrombocytosis[15].
Mast cells
editMast cells, also known as mastocytes and labrocytes, are wandering cells of connective tissues that contain many granules which are rich in histamine and heparin. They belong to granulocyte originated from myeloid stem cell which is a part of the immune system. The major function of mast cells is to defense against pathogens and participation in allergic and immunological reactions[5].
The formation of mast cell is still being debated. There is a consensus that mast cells are originated from HSCs, followed by multipotent progenitors. The subsequent stages of differentiation are still debatable. Chen et al.[16] proposed that the mast cell progenitors are mainly derived from multipotent progenitors instead of CMPs. Another model proposed by Arinobu et al.[17] suggests that CMPs give rise to granulocyte or macrophage progenitors, followed by basophil and mast cell progenitors to give mast cell progenitors. Mast cell production is believed to be highly regulated by stem cell factor[18][19].
Erythrocytes
editErythrocytes, also known as red blood cells, red cells[20], red blood cells, RBCs, haematids, erythroid cells and red blood corpuscles, are responsible for the delivery of oxygen to the body tissues via blood in the circulatory system. Red blood cells are the predominant cell type in our body, accounting for a quarter of all blood cells[21] and nearly half of the blood volume. Approximately 2.4 million red blood cells are produced per second[22] in the red bone marrow of large bones in human adults. Mature red cells have a biconcave disc shape and lack nucleus and most organelles in order to maximize oxygen and haemoglobin storing capacity[23]. The formation of red blood cells is known as erythropoiesis.
Erythropoiesis starts with a multipotent HSC, followed by different stages of differentiation. All of the stages of differentiation listed below occur in the bone marrow[5]. It starts with a multipotent HSC, followed by become a CMP or a multipotent stem cell. It further develops into a Burst-Forming Unit, Erythroid (BFU-E)[24][25], an immature erythroid progenitor. Upon maturation, it forms Colony-Forming Unit, Erythroid (CFU-E)[24][25], a more mature erythroid progenitor. This gives rise to a proerythroblast, followed by basophilic erythroblast (normoblast). Upon further development, it becomes a polychromatophilic or intermediate normoblast[2], then an orthochromatic or late normoblast (morphologically recognizable erythrocytes precursors, nucleated RBCs)[2] and finally reticulocytes.
Reticulocytes are expelled from bone marrow. They consist of about 1% of the new red blood cells in the circulation. They become mature red blood cells (erythrocytes) after 2 to 3[26] days. The general maturation process of red blood cells requires vitamin B9 (folate) and vitamin B12 (cobalamin). Insufficiency or lack of of either may lead to maturation failure, clinically known as reticulocytopenia, an abnormally low amount of reticulocytes in the body.
Macrophages
editMacrophages, which is a subtype of white blood cell, are essential for engulfment and digestion of foreign substances, cancer cells, microbes or any other things not having healthy body's immune receptor under the process of phagocytosis[27]. These large phagocytes also regulate other immune processes, for example, inflammation and recruiting other immune cells[28]. Macrophages can be found in essentially all tissues[29]. A large number of macrophages are stored in the bone marrow as “storage pools” to avoid cellular overcrowding[30].
Macrophages differentiate from monocytes and monocytes differentiate from the CFU-GM cells, a type of the precursor for monoblasts and myeloblasts.[31] Monocytes become mature in bone marrow and migrate into tissues to become macrophages. Inactivated macrophages are stored outside the bloodstream. Local signals will activate macrophages when there is injury.[32] The production of macrophages is under the regulation of Colony-Stimulating Factors (CSFs), which can promote the proliferation and differentiation of monocytes. CSFs are produced in the endothelial cells, macrophages, fibroblasts and lymphocytes[32]. Situation like bacterial infection will increase the concentration of CSFs rapidly.
Granulocytes
editGranulocytes, which is a subtype of white blood cell, are normally consisted of three lobes and multilobed nuclei. Usually there is a great amount of cytoplasmic granules within the cell, which is important for the immune system[33]. There are four types of granulocyte namely basophil, neutrophil, eosinophil and mast cell, which are responsible for cellular immune response. Among all granulocytes, neutrophil is the most abundant one compared to the other types.[34]
Granulopoiesis, as known as granulocytopoiesis, is the production of granulocytes. Basophil, neutrophil, eosinophil share a similar formation pathway. The formation of mast cell is different from other granulocytes so it is described in another section. The formation of granulocytes starts at myeloblast, a unipotent stem cell. They differentiate into promyelocyte, followed by myelocyte, then metamyelocyte, then band and finally basophil, neutrophil or eosinophil[35]. Humoral agents such as interleukin 3 and colony-stimulating factor (CSF) regulate the process of granulopoiesis.[35]. CBF, c-Myb, C/EBPα and PU.1 are four main transcription factors involving in granulopoiesis.[35] Candida albicans can be used to stimulate the process.[36].
Common lymphoid progenitors (CLPs)
editLymphocytes
editLymphocytes, a type of white blood cell, are important for the immune system. There are three major types of lymphocytes including, T cells, responding for cytotoxic adaptive immunity; B cells, responding for antibody-driven adaptive immunity; and natural killer cells, responding for cytotoxic innate immunity which is a cell-mediated process.[37].
Lymphopoiesis, also known as lymphoid hematopoiesis, is the generation of lymphocytes[38]. Pathological condition in lymphopoiesis causes various of lymphoproliferative disorders, for example lymphoid leukaemias and lymphomas.
T cells
editT cells, also known as T lymphocyte, are differentiated from haematopoietic stem cells (HSCs) in the bone marrow. During the maturation process, T cells migrate from bone marrow to the thymic cortex, an antigen-free environment. Only 2-4% of the T cells successfully undergo the process of maturation[39]. Thymocytes, also known as developing T cell, proliferate and differentiate in the thymus. The differentiation of T cell starts at naive T cell[40], then into effector cells. This differentiation is vital for the development of immune memory[40]. Licensing and instructive signals from the environment, particularly from antigen-presenting cells (APC), direct the differentiation of T cells. APCs detect the type of ingested microbe and then release signals to direct naïve T cells[41].
B cells
editB cells, as known as B lymphocytes, are differentiated from haematopoietic stem cells (HSCs) in the bone marrow[42]. HSCs first differentiate into multipotent progenitor (MPP) cells, then common lymphoid progenitor (CLP) cells. CLP cells first migrate from the bone marrow to peripheral lymphoid tissues like lymph nodes, then to the spleen. After the process of migration, they differentiate into T1 B cells[43]. T1 B cells transform to T2 B cells in the spleen eventually. After the differentiation, mature B cells, or naive B cells, are produced[44].
Natural killer (NK) cells are that are responsible for the innate protection against cancer cells and pathogens because they can release inflammatory cytokines rapidly and destroy transformed or infected cells[45]. The process of forming NK cells is still being debated. It is only clear that the differentiation and maturation of NK cells occur in the lymph nodes, bone marrow, spleen, thymus and tonsils, before entering into the circulation system[46]. There is a consensus that NK cells are differentiated from bone marrow-derived haematopoietic stem cells via the lymphoid progenitor (CLP), which can also differentiate into other lymphocyte subsets[46][47]. NK cells are mature after the acquisition of functional receptors.
See also
editReferences
edit- ^ Silva, Ariosto; Anderson, Alexander; Gatenby, Robert (2011-04). "A multiscale model of the bone marrow and hematopoiesis". Mathematical Biosciences and Engineering. 8 (2): 643–658. doi:10.3934/mbe.2011.8.643. ISSN 1551-0018.
{{cite journal}}: Check date values in:|date=(help) - ^ a b c d e f g Patel, Amit; Radia, Deepti (2017-4). "Haemopoiesis – the formation of blood cells". Medicine. 45 (4): 194–197. doi:10.1016/j.mpmed.2017.01.004.
{{cite journal}}: Check date values in:|date=(help) - ^ Morrison, Sean J.; Kimble, Judith (2006-06). "Asymmetric and symmetric stem-cell divisions in development and cancer". Nature. 441 (7097): 1068–1074. doi:10.1038/nature04956. ISSN 0028-0836.
{{cite journal}}: Check date values in:|date=(help) - ^ A., Odorico, Jon S. Zhang, S.-C. (Su-Chun) Pedersen, Roger (2005). Human embryonic stem cells. Garland Science/BIOS Scientific Publishers. ISBN 1859962785. OCLC 56672516.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ a b c Munker, Reinhold, "Basic Biology of Hemopoiesis", Contemporary Hematology, Humana Press, pp. 1–18, ISBN 9781588295576, retrieved 2019-03-12
- ^ Birbrair, Alexander; Frenette, Paul S. (2016-4). "Niche heterogeneity in the bone marrow: Cellular complexity of the HSC niche in the BM". Annals of the New York Academy of Sciences. 1370 (1): 82–96. doi:10.1111/nyas.13016. PMC 4938003. PMID 27015419.
{{cite journal}}: Check date values in:|date=(help)CS1 maint: PMC format (link) - ^ Fernández, Karen S.; de Alarcón, Pedro A. (2013-12). "Development of the Hematopoietic System and Disorders of Hematopoiesis that Present During Infancy and Early Childhood". Pediatric Clinics of North America. 60 (6): 1273–1289. doi:10.1016/j.pcl.2013.08.002.
{{cite journal}}: Check date values in:|date=(help) - ^ Laki, K. (1972-12). "OUR ANCIENT HERITAGE IN BLOOD CLOTTING AND SOME OF ITS CONSEQUENCES". Annals of the New York Academy of Sciences. 202 (1): 297–307. doi:10.1111/j.1749-6632.1972.tb16342.x. ISSN 0077-8923.
{{cite journal}}: Check date values in:|date=(help) - ^ Machlus, Kellie R.; Thon, Jonathan N.; Italiano, Joseph E. (2014-02-06). "Interpreting the developmental dance of the megakaryocyte: a review of the cellular and molecular processes mediating platelet formation". British Journal of Haematology. 165 (2): 227–236. doi:10.1111/bjh.12758. ISSN 0007-1048.
- ^ Schulman, I.; Pierce, M.; Lukens, A.; Currimbhoy, Z. (1960-7). "Studies on thrombopoiesis. I. A factor in normal human plasma required for platelet production; chronic thrombocytopenia due to its deficiency". Blood. 16: 943–957. ISSN 0006-4971. PMID 14443744.
{{cite journal}}: Check date values in:|date=(help) - ^ Jain, N. C (1975-01). "A Scanning Electron Microscopic Study of Platelets of Certain Animal Species". Thrombosis and Haemostasis. 33 (01): 501–507. doi:10.1055/s-0038-1647843. ISSN 0340-6245.
{{cite journal}}: Check date values in:|date=(help) - ^ Platelets. Michelson, Alan D. (3rd ed ed.). Amsterdam: Elsevier. 2013. ISBN 9780123878380. OCLC 820818942.
{{cite book}}:|edition=has extra text (help)CS1 maint: others (link) - ^ Kaushansky, Kenneth (2006-05-11). "Lineage-Specific Hematopoietic Growth Factors". New England Journal of Medicine. 354 (19): 2034–2045. doi:10.1056/nejmra052706. ISSN 0028-4793.
- ^ Evidence-based practice of critical care. Deutschman, Clifford S., Neligan, Patrick J. Philadelphia, PA: Saunders/Elsevier. 2010. ISBN 9781437737899. OCLC 664111280.
{{cite book}}: CS1 maint: others (link) - ^ Robbins basic pathology. Kumar, Vinay, 1944-, Robbins, Stanley L. (Stanley Leonard), 1915-2003. (8th ed ed.). Philadelphia, PA: Saunders/Elsevier. 2007. ISBN 1416029737. OCLC 69672074.
{{cite book}}:|edition=has extra text (help)CS1 maint: others (link) - ^ Chen, C.-C.; Grimbaldeston, M. A.; Tsai, M.; Weissman, I. L.; Galli, S. J. (2005-07-08). "From The Cover: Identification of mast cell progenitors in adult mice". Proceedings of the National Academy of Sciences. 102 (32): 11408–11413. doi:10.1073/pnas.0504197102. ISSN 0027-8424.
- ^ Arinobu, Y.; Iwasaki, H.; Gurish, M. F.; Mizuno, S.-i.; Shigematsu, H.; Ozawa, H.; Tenen, D. G.; Austen, K. F.; Akashi, K. (2005-12-05). "Developmental checkpoints of the basophil/mast cell lineages in adult murine hematopoiesis". Proceedings of the National Academy of Sciences. 102 (50): 18105–18110. doi:10.1073/pnas.0509148102. ISSN 0027-8424.
- ^ Okayama, Yoshimichi; Kawakami, Toshiaki (2006). "Development, Migration, and Survival of Mast Cells". Immunologic Research. 34 (2): 97–116. doi:10.1385/ir:34:2:97. ISSN 0257-277X.
- ^ Dahlin, Joakim S.; Hallgren, Jenny (2015-01). "Mast cell progenitors: Origin, development and migration to tissues". Molecular Immunology. 63 (1): 9–17. doi:10.1016/j.molimm.2014.01.018. ISSN 0161-5890.
{{cite journal}}: Check date values in:|date=(help) - ^ "Dedication", Robbins Basic Pathology, Elsevier, pp. v, 2013, ISBN 9781437717815, retrieved 2019-03-13
- ^ Pierigè, F.; Serafini, S.; Rossi, L.; Magnani, M. (2008-01). "Cell-based drug delivery". Advanced Drug Delivery Reviews. 60 (2): 286–295. doi:10.1016/j.addr.2007.08.029. ISSN 0169-409X.
{{cite journal}}: Check date values in:|date=(help) - ^ Sackmann, E. (1995), "Biological Membranes Architecture and Function", Handbook of Biological Physics, Elsevier, pp. 1–63, ISBN 9780444819758, retrieved 2019-03-13
- ^ Strauss, Dirk C; Botha, Abraham J; Taylor, Irving, "Haemopoietic and lymphoreticular systems: anatomy, physiology and pathology", Fundamentals of Surgical Practice, Cambridge University Press, pp. 199–229, ISBN 9780511545740, retrieved 2019-03-13
- ^ a b Elliott, Steve; Pham, Elizabeth; Macdougall, Iain C. (2008-12). "Erythropoietins: A common mechanism of action". Experimental Hematology. 36 (12): 1573–1584. doi:10.1016/j.exphem.2008.08.003. ISSN 0301-472X.
{{cite journal}}: Check date values in:|date=(help) - ^ a b Hattangadi, S. M.; Wong, P.; Zhang, L.; Flygare, J.; Lodish, H. F. (2011-10-12). "From stem cell to red cell: regulation of erythropoiesis at multiple levels by multiple proteins, RNAs, and chromatin modifications". Blood. 118 (24): 6258–6268. doi:10.1182/blood-2011-07-356006. ISSN 0006-4971.
- ^ Ney, Paul A (2011-05). "Normal and disordered reticulocyte maturation". Current Opinion in Hematology. 18 (3): 152–157. doi:10.1097/moh.0b013e328345213e. ISSN 1065-6251.
{{cite journal}}: Check date values in:|date=(help) - ^ Mlynski, Gunter H. (2013), "Physiology and Pathophysiology of Nasal Breathing", Nasal Physiology and Pathophysiology of Nasal Disorders, Springer Berlin Heidelberg, pp. 77–88, ISBN 9783642372490, retrieved 2019-03-13
- ^ Hu, Kebin; Jin, Yang; Chroneos, Zissis; Han, Xiaodong; Liu, Hao; Lin, Ling (2018-06-05). "Macrophage Functions and Regulation: Roles in Diseases and Implications in Therapeutics". Journal of Immunology Research. 2018: 1–2. doi:10.1155/2018/7590350. ISSN 2314-8861.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Ovchinnikov, Dmitry A. (2008-09). "Macrophages in the embryo and beyond: Much more than just giant phagocytes". genesis. 46 (9): 447–462. doi:10.1002/dvg.20417. ISSN 1526-954X.
{{cite journal}}: Check date values in:|date=(help) - ^ Okabe, Yasutaka; Medzhitov, Ruslan (2016-04). "Wormhole Travel for Macrophages". Cell. 165 (3): 518–519. doi:10.1016/j.cell.2016.04.005. ISSN 0092-8674.
{{cite journal}}: Check date values in:|date=(help) - ^ "Editors", xPharm: The Comprehensive Pharmacology Reference, Elsevier, p. 1, 2008, ISBN 9780080552323, retrieved 2019-03-13
- ^ a b "Leukopoiesis", Encyclopedic Reference of Molecular Pharmacology, Springer-Verlag, pp. 551–551, ISBN 3540428437, retrieved 2019-03-13
- ^ "Innate Immune System", SpringerReference, Springer-Verlag, retrieved 2019-03-13
- ^ "Polymorphonuclear Leukocyte", SpringerReference, Springer-Verlag, retrieved 2019-03-13
- ^ a b c Ward, AC; Loeb, DM; Soede-Bobok, AA; Touw, IP; Friedman, AD (2000-06). "Regulation of granulopoiesis by transcription factors and cytokine signals". Leukemia. 14 (6): 973–990. doi:10.1038/sj.leu.2401808. ISSN 0887-6924.
{{cite journal}}: Check date values in:|date=(help) - ^ Basu, Sunanda; Zhang, Hui-Hua; Quilici, Cathy; Dunn, Ashley R. (2004-02). "Candida albicans Can Stimulate Stromal Cells Resulting in Enhanced Granulopoiesis". Stem Cells and Development. 13 (1): 39–50. doi:10.1089/154732804773099245. ISSN 1547-3287.
{{cite journal}}: Check date values in:|date=(help) - ^ "Cells and Organs of the Immune System", Immunology for Pharmacy, Elsevier, pp. 1–14, 2012, ISBN 9780323069472, retrieved 2019-03-13
- ^ Yu, Vionnie W.C.; Scadden, David T. (2016-07). "Heterogeneity of the bone marrow niche". Current Opinion in Hematology. 23 (4): 331–338. doi:10.1097/moh.0000000000000265. ISSN 1065-6251.
{{cite journal}}: Check date values in:|date=(help) - ^ OA Molecular and Cell Biology. Open Access Publishing London.
- ^ a b Frederick., Alt, (2018). Advances in Immunology. Elsevier Science & Technology. ISBN 9780128155271. OCLC 1061126474.
{{cite book}}: CS1 maint: extra punctuation (link) CS1 maint: multiple names: authors list (link) - ^ Vantourout, Pierre; Hayday, Adrian (2013-2). "Six-of-the-best: unique contributions of γδ T cells to immunology". Nature Reviews Immunology. 13 (2): 88–100. doi:10.1038/nri3384. ISSN 1474-1733. PMC 3951794. PMID 23348415.
{{cite journal}}: Check date values in:|date=(help)CS1 maint: PMC format (link) - ^ Chung, James B.; Silverman, Michael; Monroe, John G. (2003-6). "Transitional B cells: step by step towards immune competence". Trends in Immunology. 24 (6): 342–348. doi:10.1016/S1471-4906(03)00119-4.
{{cite journal}}: Check date values in:|date=(help) - ^ Loder, By Florienne; Mutschler, Bettina; Ray, Robert J.; Paige, Christopher J.; Sideras, Paschalis; Torres, Raul; Lamers, Marinus C.; Carsetti, Rita (1999-07-01). "B Cell Development in the Spleen Takes Place in Discrete Steps and Is Determined by the Quality of B Cell Receptor–Derived Signals". The Journal of Experimental Medicine. 190 (1): 75–90. doi:10.1084/jem.190.1.75. ISSN 0022-1007.
- ^ Yu, Jianhua; Freud, Aharon G.; Caligiuri, Michael A. (2013-12). "Location and cellular stages of natural killer cell development". Trends in Immunology. 34 (12): 573–582. doi:10.1016/j.it.2013.07.005. ISSN 1471-4906.
{{cite journal}}: Check date values in:|date=(help) - ^ Iannello, Alexandre; Debbeche, Olfa; Samarani, Suzanne; Ahmad, Ali (2008-7). "Antiviral NK cell responses in HIV infection: I. NK cell receptor genes as determinants of HIV resistance and progression to AIDS". Journal of Leukocyte Biology. 84 (1): 1–26. doi:10.1189/jlb.0907650.
{{cite journal}}: Check date values in:|date=(help) - ^ a b Blom, Bianca; Spits, Hergen (2006-4). "DEVELOPMENT OF HUMAN LYMPHOID CELLS". Annual Review of Immunology. 24 (1): 287–320. doi:10.1146/annurev.immunol.24.021605.090612. ISSN 0732-0582.
{{cite journal}}: Check date values in:|date=(help) - ^ Kondo, M.; Scherer, D. C.; King, A. G.; Manz, M. G.; Weissman, I. L. (2001-10). "Lymphocyte development from hematopoietic stem cells". Current Opinion in Genetics & Development. 11 (5): 520–526. ISSN 0959-437X. PMID 11532393.
{{cite journal}}: Check date values in:|date=(help)