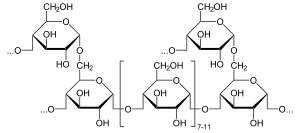

Alpha-glucosidase (EC 3.2.1.20 ) is a glucosidase that acts upon 1,4-alpha bonds.[1] This is in contrast to beta-glucosidase. Alpha-glucosidase breaks down starch and disaccharides to glucose.Maltase, a similar enzyme that cleaves maltose, is nearly functionally equivalent.
Other glucosidases include:
Mechanism
editAlpha-glucosidase hydrolyzes terminal non-reducing 1-4 linked alpha- glucose residues to release a single alpha- glucose molecule.[2] Alpha-glucosidase is a carbohydrate-hydrolase that releases alpha-glucose as opposed to beta-glucose. Beta-glucose residues can be released by glucoamylase, a functionally similar enzyme. The substrate selectivity of alpha-glucosidase is due to subsite affinities of the enzyme’s active site. [3] Two proposed mechanisms include a nucleophilic displacement and a oxocarbenium ion intermediate.[3]
- Rhodnius prolixus, a blood-sucking insect, forms hemozoin (Hz) during digestion of host hemoglobin. Hemozoin synthesis is dependent on the substrate binding site of alpha-glucosidase. [4]
- Trout liver alpha-glucosidases were extracted and characterized. It was shown that for one of the trout liver alpha-glucosidases maximum activity of the enzyme was increased by 80% during exercise in comparison to a resting trout. This change was shown to correlate to an activity increase for liver glycogen phosphorylase. It is proposed that alpha-glucosidase in the glucosidic path plays an important part in complementing the phosphorolytic pathway in the liver’s metabolic response to energy demands of exercise.[5]
- Yeast and rat small intestinal alpha-glucosidases have been shown to be inhibited by several groups of flavonoids. [6]
Structure
editAlpha-glucosidases can potentially be split, according to primary structure, into two families. [3] The gene coding for human lysosomal alpha-glucosidase is about 20 kb long and it’s structure has been cloned and confirmed. [7]
- Human lysosomal alpha-glucosidase has been studied for the significance of the Asp-518 and other residues in proximity of the enzyme’s active site. It was found that substituting Asp-513 with Glu-513 interferes with posttranslational modification and intracellular transport of alpha-glucosidase’s precursor. Additionally, the Trp-516 and Asp-518 residues have been deemed critical for the enzyme’s catalytic functionality. [8]
- Kinetic changes in alpha-glucosidase have been shown to be induced by denaturants such as guanidinium chloride (GdmCl) and SDS solutions. These denaturants cause loss of activity and conformational change. A loss of enzyme activity occurs at much lower concentrations of denaturant than required for conformational changes. This leads to a conclusion that the enzyme’s active site conformation is less stable than the whole enzyme conformation in response to the two denaturants. [9]
Disease Relevance
edit- Pompe Disease: a disorder in which alpha-glucosidase is deficient. In 2006, the drug Alglucosidase alfa became the first released treatment for Pompe Disease and acts as an analog to alpha-glucosidase. [10]Further studies of alglucosidase alfa revealed that imino sugars exhibit inhibition of the enzyme. It was found that one compound molecule binds to a single enzyme molecule. It was shown that 1-deoxynojirimycin (DNJ) would bind the strongest of the sugars tested and blocked the active site of the enzyme almost entirely. The studies enhanced knowledge of the mechanism by which alpha-glucosidase binds to imino sugars. [11]
- Diabetes: Luteolin has been found to be a strong inhibitor of alpha-glucosidase. The compound can inhibit the enzyme up to 36% with a concentration of 0.5 mg/ml. These results hint that luteolin has potential to suppress postprandial hyperglycemia in non-insulin dependent diabetes mellitus patients and there is value in pursuing a greater understanding of luteolin’s potential for treatment.[12] Acarbose, another alpha-glucosidase inhibitor competitively and reversibly inhibits alpha-glucosidase in the intestines. This inhibition lowers the rate of glucose absorption through delayed carbohydrate digestion and extended digestion time. It has been determined that acarbose may have the capability to permanently or temporarily stop developing diabetic symptoms. [13]
Hence, alpha-glucosidase inhibitors (like Acarbos), are used as anti-diabetic drugs in combination with other anti-diabetic drugs.
- Azoospermia: Diagnosis of azoospermia has potential to be aided by measurement of alpha-glucosidase activity in seminal plasma. Activity in the seminal plasma corresponds to the functionality of the epididymis. [14]
- Anti-Viral Agents: Many animal viruses possess an outer envelope comprised of viral glycoproteins. These are often required for the viral life cycle and utilize cellular machinery for synthesis. Inhibitors of alpha-glucosidase show that the enzyme is involved in the pathway for N-glycans for viruses such as HIV and human hepatitis B virus (HBV). Inhibition of alpha-glucosidase can prevent fusion of HIV and secretion of HBV. [15]
See also
edit
Hence, α-glucosidase inhibitors (like Acarbos) are used as anti-diabetic drugs in combination with other anti-diabetic drugs.
References
edit- ^ alpha-Glucosidases at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- ^ "EC 3.2.1.20". ExPASy. Retrieved 1 March 2012.
- ^ a b c Chiba S (August 1997). "Molecular mechanism in alpha-glucosidase and glucoamylase". Biosci. Biotechnol. Biochem. 61 (8): 1233–9. doi:10.1271/bbb.61.1233. PMID 9301101.
{{cite journal}}: CS1 maint: date and year (link) - ^ Mury FB, da Silva JR, Ferreira LS; et al. (2009). "Alpha-glucosidase promotes hemozoin formation in a blood-sucking bug: an evolutionary history". PLOS ONE. 4 (9): e6966. doi:10.1371/journal.pone.0006966. PMC 2734994. PMID 19742319.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Mehrani H, Storey KB (October 1993). "Characterization of alpha-glucosidases from rainbow trout liver". Arch. Biochem. Biophys. 306 (1): 188–94. doi:10.1006/abbi.1993.1499. PMID 8215402.
{{cite journal}}: CS1 maint: date and year (link) - ^ Tadera K, Minami Y, Takamatsu K, Matsuoka T (April 2006). "Inhibition of alpha-glucosidase and alpha-amylase by flavonoids". J. Nutr. Sci. Vitaminol. 52 (2): 149–53. doi:10.3177/jnsv.52.149. PMID 16802696.
{{cite journal}}: CS1 maint: date and year (link) CS1 maint: multiple names: authors list (link) - ^ Hoefsloot, L. H.; Hoogeveen-Westerveld, M.; Reuser, A. J.; Oostra, B. A. (1). "Characterization of the human lysosomal alpha-glucosidase gene". Biochem J. 272 (2): 493–497. doi:10.1042/bj2720493. PMC 1149727. PMID PMC1149727.
{{cite journal}}: Check|pmid=value (help); Check date values in:|date=and|year=/|date=mismatch (help); Unknown parameter|month=ignored (help) - ^ Hermans, Monique (25). "Human Lysosomal a-Glucosidase Characterization of The Catalytic Site". The Journal of Biological Chemistry. 21. 266: 13507–13512. doi:10.1016/S0021-9258(18)92727-4. Retrieved 1 March 2012.
{{cite journal}}: Check date values in:|date=and|year=/|date=mismatch (help); Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ Wu XQ, Xu H, Yue H, Liu KQ, Wang XY (December 2009). "Inhibition kinetics and the aggregation of alpha-glucosidase by different denaturants". Protein J. 28 (9–10): 448–56. doi:10.1007/s10930-009-9213-0. PMID 19921411.
{{cite journal}}: CS1 maint: date and year (link) CS1 maint: multiple names: authors list (link) - ^ "FDA Approves First Treatment for Pompe Disease". FDA News Release. FDA. Retrieved 1 March 2012.
- ^ Yoshimizu, M. (May 2008). "Binding parameters and thermodynamics of the interaction of imino sugars with a recombinant human acid alpha-glucosidase (alglucosidase alfa): insight into the complex formation mechanism". Clin Chim Acta. 391 (1–2): 68–73. doi:10.1016/j.cca.2008.02.014. PMID 18328816.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help)CS1 maint: date and year (link) - ^ Kim JS, Kwon CS, Son KH (November 2000). "Inhibition of alpha-glucosidase and amylase by luteolin, a flavonoid". Biosci. Biotechnol. Biochem. 64 (11): 2458–61. doi:10.1271/bbb.64.2458. PMID 11193416.
{{cite journal}}: CS1 maint: date and year (link) CS1 maint: multiple names: authors list (link) - ^ Bischoff H (August 1995). "The mechanism of alpha-glucosidase inhibition in the management of diabetes". Clin Invest Med. 18 (4): 303–11. PMID 8549017.
{{cite journal}}: CS1 maint: date and year (link) - ^ Mahmoud AM, Geslevich J, Kint J; et al. (March 1998). "Seminal plasma alpha-glucosidase activity and male infertility". Hum. Reprod. 13 (3): 591–5. doi:10.1093/humrep/13.3.591. PMID 9572418.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: date and year (link) CS1 maint: multiple names: authors list (link) - ^ Mehta, Anand (23). "α-Glucosidase inhibitors as potential broad based anti-viral agents". FEBS Letters. 430 (1–2): 17–22. doi:10.1016/S0014-5793(98)00525-0. PMID 9678587. Retrieved 1 March 2012.
{{cite journal}}: Check date values in:|date=and|year=/|date=mismatch (help); Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help)
