The following articles are featured as the Selected intervention at the Viruses Portal. To suggest an article for inclusion, use the suggestions page
Portal:Viruses/Selected intervention/1
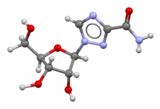
Zidovudine (ZDV) (also known as AZT and sold as Retrovir) is an antiretroviral drug used in the prevention and treatment of HIV/AIDS. Classed as a nucleoside analogue reverse-transcriptase inhibitor, it inhibits HIV's reverse transcriptase enzyme, which copies the viral RNA into DNA and is essential for its replication. The first breakthrough in AIDS therapy, ZDV was licensed in 1987. While it significantly reduces HIV replication, leading to some clinical and immunological benefits, when used alone ZDV does not completely stop replication, allowing the virus to become resistant to it. The drug is therefore used together with other anti-HIV drugs in combination therapy called highly active antiretroviral therapy. To simplify its administration, ZDV is included in combination pills with lamivudine (Combivir) and lamivudine plus abacavir (Trizivir). ZDV continues to be used to prevent HIV transmission from mother to child during childbirth; it was previously part of the standard post-exposure prophylaxis after needlestick injury.
Portal:Viruses/Selected intervention/2
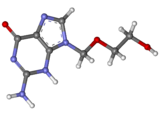
Aciclovir (also acyclovir and sold as Zovirax) is a nucleoside analogue that mimics the nucleoside guanosine. It is active against most viruses in the herpesvirus family, and is mainly used to treat herpes simplex virus infections, chickenpox and shingles. After phosphorylation by viral thymidine kinase and cellular enzymes, the drug inhibits the viral DNA polymerase. Extremely selective and low in cytotoxicity, it was seen as the start of a new era in antiviral therapy. Aciclovir was discovered by Howard Schaeffer and colleagues, and developed by Schaeffer and Gertrude Elion, who was awarded the 1988 Nobel Prize in Medicine in part for its development. Nucleosides isolated from a Caribbean sponge, Cryptotethya crypta, formed the basis for its synthesis. Aciclovir differs from earlier nucleoside analogues in containing only a partial nucleoside structure: the sugar ring is replaced with an open chain. Resistance to the drug is rare in people with a normal immune system.
Portal:Viruses/Selected intervention/3
Two polio vaccines are used against the paralytic disease polio. The first, developed by Jonas Salk, consists of inactivated poliovirus. Based on three wild virulent strains, inactivated using formalin, it is administered by injection and is very safe. It confers IgG-mediated immunity, which prevents poliovirus from entering the bloodstream and protects the motor neurons, eliminating the risk of bulbar polio and post-polio syndrome. The second, developed by Albert Sabin, originally consisted of three live virus strains, attenuated by growth in cell culture. Since 2016, only two strains have generally been included. They contain multiple mutations, preventing them from replicating in the nervous system. The Sabin vaccine stimulates both antibodies and cell-mediated immunity, providing longer-lasting immunity than the Salk vaccine. It can be administered orally, making it more suitable for mass vaccination campaigns. In around three cases per million doses, the live vaccine reverts to a virulent form and causes paralysis. Vaccination has reduced the number of wild-type polio cases from around 350,000 in 1988 to just 33 in 2018, and eradicated the disease from most countries.
Portal:Viruses/Selected intervention/4
| “ | the most damaging medical hoax of the last 100 years —Dennis Flaherty, 2011 |
” |
The MMR vaccine and autism fraud refers to the false claim that the combined vaccine for measles, mumps and rubella (MMR) might be associated with colitis and autism spectrum disorders. Multiple large epidemiological studies have since found no link between the vaccine and autism. The notion originated in a fraudulent research paper by Andrew Wakefield and co-authors, published in the prestigious medical journal The Lancet in 1998. Sunday Times journalist Brian Deer's investigations revealed that Wakefield had manipulated evidence and had multiple undeclared conflicts of interest. The paper was retracted in 2010, when the Lancet's editor-in-chief Richard Horton characterised it as "utterly false". Wakefield was found guilty of serious professional misconduct by the General Medical Council, and struck off the UK's Medical Register. The claims in Wakefield's article were widely reported in the press, resulting in a sharp drop in vaccination uptake in the UK and Ireland. A greatly increased incidence of measles and mumps followed, leading to deaths and serious permanent injuries.
Portal:Viruses/Selected intervention/5
Ribavirin is a nucleoside analogue that mimics the nucleoside guanosine. It shows some activity against a broad range of DNA and RNA viruses, but is less effective against dengue fever, yellow fever and other flaviviruses. The drug was first synthesised in the early 1970s by Joseph T. Witkowski and Roland K. Robins. Ribavirin's main current use is against hepatitis C, in combination with pegylated interferon, nucleotide analogues and protease inhibitors. It has been used in the past in an aerosol formulation against respiratory syncytial virus-related diseases in children. Ribavirin has been used in combination as part of an experimental treatment for rabies. It is also the only available treatment for the viruses causing some viral haemorrhagic fevers, including Lassa fever, Crimean–Congo haemorrhagic fever and hantavirus disease, but is ineffective against the filovirus diseases, Ebola and Marburg. Clinical use is limited by the drug building up in red blood cells to cause haemolytic anaemia.
Portal:Viruses/Selected intervention/6
Several human papillomavirus (HPV) vaccines, including Cervarix and Gardasil, have been approved to protect against infections with particular types of HPV, associated with cervical and other cancers. All vaccines protect against the high-risk HPV types 16 and 18. Gardasil is a quadrivalent vaccine that additionally protects against low-risk HPV-6 and -11, which are associated with most cases of genital warts. A second-generation nine-valent Gardasil vaccine protects against five additional high-risk HPV types. It is estimated that the vaccines may prevent 70% of cervical cancer, 80% of anal cancer, 60% of vaginal cancer, 40% of vulvar cancer and possibly some oropharyngeal cancers. Protection lasts for at least 8–9 years. Some advocate giving Gardasil to men and boys with the primary aim of protecting their female sexual partners; others consider vaccinating only women and girls to be more cost effective. The licensed vaccines are subunit vaccines, containing only the L1 capsid protein of the virus, which self-assembles into virus-like particles. They are not effective in people already infected with HPV. Research is ongoing into therapeutic HPV vaccines including the viral oncoproteins, E6 and E7, but none has yet been licensed.
Portal:Viruses/Selected intervention/7
Nevirapine (also Viramune) is an antiretroviral drug used in the treatment of HIV/AIDS caused by HIV-1. It was the first non-nucleoside reverse transcriptase inhibitor to be licensed, which occurred in 1996. Like nucleoside inhibitors, nevirapine inhibits HIV's reverse transcriptase enzyme, which copies the viral RNA into DNA and is essential for its replication. Unlike nucleoside inhibitors, it binds not in the enzyme's active site but in a nearby hydrophobic pocket, causing a conformational change in the enzyme that prevents it from functioning. Mutations in the pocket generate resistance to nevirapine, which develops rapidly unless viral replication is completely suppressed. The drug is therefore only used together with other anti-HIV drugs in combination therapy. The HIV-2 reverse transcriptase has a different pocket structure, rendering it inherently resistant to nevirapine and other first-generation NNRTIs. A single dose of nevirapine is a cost-effective way to reduce mother-to-child transmission of HIV, and has been recommended by the World Health Organization for use in resource-poor settings. Other protocols are recommended in the United States. Rash is the most common adverse event associated with the drug.
Portal:Viruses/Selected intervention/8
Oseltamivir (also Tamiflu) is an oral antiviral drug against influenza (flu). It was the second inhibitor of the viral neuraminidase to be developed, after zanamivir, and the first to be taken as an oral tablet. It was originally synthesised from shikimic acid extracted from the star anise plant. Oseltamivir is a prodrug that requires metabolism in the liver to the active form, oseltamivir carboxylate. This binds at the active site of the neuraminidase enzyme, preventing it from cleaving sialic acid to release the virus particle from the host cell. Oseltamivir can reduce the duration of influenza symptoms by 0.5–1 days. Debate is ongoing about whether it also reduces the risk of complications, such as pneumonia. Nausea and vomiting are the main adverse events. Resistance to oseltamivir has been observed in some strains of influenza virus, especially H1N1 strains.
Portal:Viruses/Selected intervention/9
Influenza vaccines include live attenuated and inactivated forms. Inactivated vaccines contain three or four different viral strains selected by the World Health Organization to cover influenza A H1N1 and H3N2, as well as influenza B, and are usually administered by intramuscular injection. The live attenuated influenza vaccine contains a cold-adapted strain and is given as a nasal spray. Most influenza vaccine strains are cultivated in fertilised chicken eggs (pictured), a technique developed in the 1950s; others are grown in cell cultures, and some vaccines contain recombinant proteins. Annual vaccination is recommended for high-risk groups and, in some countries, for all those over six months. As the influenza virus changes rapidly by antigenic drift, new versions of the vaccine are developed twice a year, which differ in effectiveness depending on how well they match the circulating strains. Despite considerable research effort for decades, no effective universal influenza vaccine has been identified. A 2018 meta-analysis found that vaccination in healthy adults decreased confirmed cases of influenza from about 2.4% to 1.1%. However, the effectiveness is uncertain in those over 65 years old, one of the groups at highest risk of serious complications.
Portal:Viruses/Selected intervention/10
The first Ebola vaccine was approved in 2019. Developed by the Public Health Agency of Canada, rVSV-ZEBOV is based on an attenuated recombinant vesicular stomatitis virus, genetically modified to express a surface glycoprotein of Zaire ebolavirus, and is estimated to be 97.5% effective. In the Kivu Ebola epidemic of 2018–20, a ring vaccination strategy was employed to protect direct and indirect contacts of infected people, as well as health workers, and around 300,000 people were vaccinated with rVSV-ZEBOV. A second vaccine was approved in 2020; this uses two different doses – a vector based on human adenovirus serotype 26 used to prime, boosted around eight weeks later by modified vaccinia Ankara (based on a heavily attenuated vaccinia virus) – and is not suitable for response to an outbreak. The efficacy is unknown. Multiple other vaccine candidates are in development to prevent Ebola, including replication-deficient adenovirus vectors, replication-competent human parainfluenza 3 vectors, and virus-like nanoparticle preparations.
Portal:Viruses/Selected intervention/11
Portal:Viruses/Selected intervention/11
Portal:Viruses/Selected intervention/12
Portal:Viruses/Selected intervention/12