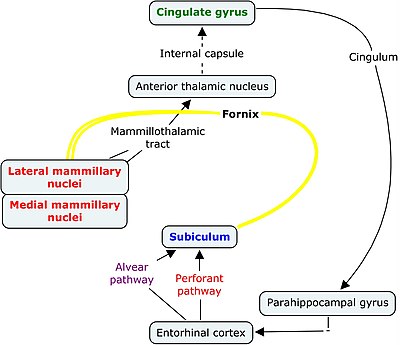
The Papez circuit, first discovered by James Papez in 1937, is a circuit within the brain that consolidates memories into the long-term store. This loop runs from the association cortex to the cingulate gyrus and hippocampal formation onto the amygdala and mammillary bodies, via the fornix; then onto the hypothalamus; from the mammillary bodies to the anterior thalamic nuclei via the mammillothalamic tract; back to the cingulate gyrus and the prefrontal cortex. It is more commonly associated with declarative memory rather than procedural memory. Damage to the Papez circuit will lead to insufficient consolidation of memories. Memory related diseases, such as Korsakoff syndrome, Kluver-Bucy syndrome and Alzheimer’s disease have all shown to have an effect on the Papez circuit.
History edit
James Papez (1937) edit
The Papez Circuit was first proposed by James Papez in 1937 in a paper titled ‘’A proposed mechanism of emotion’’[1]. He suggested that the circuit was essential for emotional experience and expression. Papez injected the rabies virus into the hippocampus of a cat, and monitored its advancement through the brain. The rabies virus was studied as this disease is known to cause heightened levels of aggression. From his experiment, Papez identified that increased levels of aggression correlated with damage to the hippocampus, as well as other brain structures. Furthermore, he theorised that these findings infer the hippocampus plays a role in emotional expression, based on its connection to the autonomic nervous system. Additional experiments involving an array of stimuli (i.e. smell and taste) were also shown to produce an emotional response in not only the hippocampus but several other regions within the brain. Collectively Papez concluded that these brain regions form a closed circuit within the brain which he believed essential for emotion; the Papez circuit. This circuit consisted of: the hippocampal formation (in particular the hippocampus), the mammillary bodies, anterior thalamic nucleus and the cingulate gyrus. At the time of publication Papez reported ‘no negative or contradictory evidence’; however he explicitly stated that he has no clinical evidence to hold this view. Instead his assumptions were based on theories proposed by Cannon [2], Bard [3] and Dandy [4]. Papez claimed his ideas sufficiently met the physiological requirements proposed by the aforementioned researchers. These theories suggested that emotional processes are located within specific structures including the diencephalon regions, and somewhere near the midline of the brain.
Maclean’s Limbic System (1952) edit
In 1952 Maclean expanded on Papez ideas to develop what is now known as the limbic system [5] [6]. The system is thought to include, but is not limited to, the hippocampus and associated structures, septal nuclei and limbic lobe. The limbic system is thought to play a role in emotion, motivation, behaviour, long-term memory, learning, and olfaction.
Current View edit
In light of Maclean’s findings the original Papez circuit has been updated to incorporate several other regions into the circuit. These include the prefrontal cortex, association cortex, amygdala and the hypothalamus. The circuit is now thought to be one of the major pathways of the limbic system, with current opinions suggesting the Papez circuit has little involvement in relation to emotion, and rather it is believed to be intrinsically involved in long-term memory.
Main Components: edit
The original Papez circuit consisted of; [7].
Hippocampal formation → via the fornix → mammillary bodies;
Mammillary bodies → via the mamillothalamic tract → anterior thalamic nuclei;
Anterior thalamic nuclei → projects to the cingulate gyrus;
cingulate gyrus → via the entorhinal cortex → hippocampal formation.
Hippocampal Formation edit
The term hippocampal formation refers to the dentate gyrus, hippocampus (subiculum) and the parahippocampal gyrus[8]. It is located within the medial temporal lobe of both cerebral hemispheres, and is thought to play a major role in memory, as well as attentional control and spatial navigation [9]. Today’s consensus suggests there is little support for the structure’s direct role in emotion [10]. The main projection of the hippocampal formation is to the mammillary bodies, via the fornix; whilst afferents are received from the cingulate gyrus projecting to the entorhinal cortex of the parahippocampal gyrus [11].
Mammillary Bodies edit
The mammillary bodies are a pair of small globular bodies located at the end of the fornix, and are thought to be involved in the processing of recognition memory [12]. They receive information from the hippocampus via the fornix and amygdala, and project to the anterior thalamic nuclei via the mammillothalamic tract [13].
Anterior Thalamic Nuclei edit
The anterior thalamic nuclei are located at the rostral end of the dorsal thalamus. They are thought to play a role in modulating alertness, and in learning and memory. The anterior thalamic nuclei receive afferents from the mammillary bodies via the mamillothalamic tract and the subiculum via the fornix. They then project to the cingulate gyrus [14].
Cingulate Gyrus edit
The cingulate gyrus is located directly above the corpus callosum. This structure is thought to be a fundamental part of both the limbic system and the Papez circuit, involved in learning and memory along with emotion formation and processing. The cingulate gyrus receives afferents from the thalamus (including the anterior thalamic nuclei) and projects to the entorhinal cortex via the cingulum, and onto the parahippocampal gyrus of the hippocampal formation; creating a great ‘loop’. [15]
Main Subcortical Connections edit
Fornix edit
The fornix is a c-shaped tract that connects the hippocampus to the mammillary bodies of the hypothalamus. The fornix extends from the hippocampus and arcs up to the anterior commissure where it splits into three parts; the precommissural fornix, the anterior commissure fornix and the postcommissural fornix (see fornix of the brain). The branch pivotal to the Papez circuit is the postcommissural fornix, which connects the hippocampus to the mammillary bodies. [16] Damage to the fornix can cause impairments of temporal order memory for retrograde information [17]. For example, patients with damage to the fornix are able to describe a holiday that occurred one year ago, and five years previously. However, they could not decide which event took place first or second.
Mammillothalamic tract edit
The mammillothalamic tract (see mammillothalamic fasciculus) ascends from both the fibres of the fornix and cells within the mammillary bodies. The mammillothalamic tract then connects to the dorsal tegmental nuclei, ventral tegmental nuclei and the anterior thalamic nuclei (see thalamic nuclei) [18] . The hippocampal-anterior thalamic pathway is thought to be essential for episodic memory. Damage to the mamillothalamic tract is linked to amnesia and Korsakoff's syndrome [19].Gold and Squire investigated a patient called PN, who had suffered from damage to the mammillary nuclei, mamillothalamic tract and anterior thalamic nuclei. Following this damage PN showed normal recognition memory and priming, however he displayed severely impaired declarative memory (i.e. both anterograde amnesia and retrograde amnesia).
Updated Circuit edit
When Maclean discovered the limbic system [20], it was suggested that the original Papez circuit needed updating to include these other connections outside of the great loop. This suggestion was based on the fact that the original Papez circuit did not include regions now thought to be essential for emotion (e.g., amygdala), rather the regions of the original circuit were more cognitive based (e.g., memory). The current state of research implies that even with the new additions of areas essential for emotion, the Papez circuit is primarily involved in memory. [21]. The updated circuit includes: Amygdala, hypothalamus, prefrontal cortex and association cortex.
Amygdala edit
The amygdalae are almond shaped nuclei located within the medial temporal lobes. The role of the amygdala is thought to be central for emotional reactions, emotional behaviour & motivation, and the processing of memories, in particular negative memories. [22]The amygdala receives afferents from all senses (i.e., olfactory, auditory, visual and somatosensory), and sends efferent’s to the hypothalamus and hippocampal formation, more specifically the hippocampus and the entorhinal cortex. [23] The original Papez circuit was missing this ‘emotional’ component. Although the updated Papez circuit is currently not considered to be intrinsically involved in emotion (unlike the limbic system), it is vital that this emotional centre is incorporated into the Papez circuit. This is because emotion can significantly affect the way memories are produced and stored. You are more likely to remember an emotionally significant event, for example most people will be able to vividly recall a birthday of theirs, even from their childhood.
Hypothalamus edit
The hypothalamus is located above the brain stem and below the thalamus, forming the ventral part of the diencephalon. It has many functions including, fatigue, circadian cycles, thirst, hunger and body temperature. The hypothalamus receives inputs from, and projects to, a vast amount of regions. In relation to the Papez circuit these include inputs from the anterior hypothalamic nucleus and the mammillary bodies, and projections to the amygdala, mamillothalamic tract and the fornix. [24]
Prefrontal Cortex edit
The prefrontal cortex is located in front of the motor and premotor areas (see frontal lobe), forming the anterior part of the frontal lobe. The prefrontal cortex as a whole is implicated in complex cognitive behaviours known as executive functions; such as decision making, expression of personality, short term memory, and moderating social behaviour. [25]. The prefrontal cortex receives inputs from, and projects to, a vast amount of regions. In relation to the Papez circuit these include connections with the amygdala, cingulate gyrus, hippocampal formation and the mediodorsal nucleus of the thalamus [26] Goldman-Rakic, Selemon and Schwarts (1984) emphasise a multi-channel communication link between the prefrontal cortex and the hippocampus. In particular they suggest that prefrontal projections to the parahippocampal gyrus and subiculum carrying highly specific information. These bi-directional projections also allow the prefrontal cortex to retrieve memories stored by the hippocampus [27]
Association Cortex edit
The association cortex, (see association area), refers to any part of the cerebral cortex outside of the primary areas. It is responsible for the integration of sensory information and the production of behaviour. [28]The association cortex receives inputs from, and projects to, a vast amount of regions. In relation to the Papez circuit these include inputs from the thalamus, and outputs which reach the hippocampus, thalamus and other association cortices. The association cortex therefore has a bidirectional link with components that form the Papez circuit. The association cortex can be split into three cortices; parietal, temporal and frontal (see association area). Research suggests the function of the temporal association cortex is important for recognition memory, such as identifying the nature of stimuli. [29]
Function of the Papez circuit edit
Consolidation of Long Term Memory edit
Long term memory is divided into two types of memory: declarative and procedural. Procedural memory refers to unconscious memories such as priming, simple forms of conditioning, skills and habits [30] . The mechanisms involved in procedural memory are not thought to be contingent with the Papez circuit. Declarative memory refers to conscious recall of facts (semantic memories) and events (episodic memory) [31]. [32]. Studies with amnesic patients who have suffered damaged to the Papez circuit, such as Patient HM have shown that patients have intact procedural memory, but impaired declarative memory. The Papez circuit can be imagined as the ‘glue’ that holds memories together. For example, when you first see a face and hear a name at the same time, the Papez circuit will consolidate those two pieces of information (i.e., auditory and visual) together. This is stored in long term memory. Repeated exposure to both stimuli, will lead to a fully consolidated memory. Note that long-term memories are not stored within the structures of the Papez circuit, they are simply retrieved from their original storage through the mechanisms of the Papez circuit. Damage to the Papez circuit will result in insufficient consolidation, therefore not allowing the memory to be transferred to the long term store. Research suggests that fully consolidated memories no longer rely on the Papez circuit. [33] These conclusions are heavily based on research with amnesic patients [34].
Associated Syndromes edit
Amnesia edit
There are two types of amnesia: anterograde and retrograde. Anterograde amnesia is where a person is unable to remember on going events after a head trauma. A sufferer of anterograde amnesia will not tend to forget who they are, or anything previous to the incident; however they will have severe difficulty remembering anything new. Retrograde amnesia is where a person is able to remember things after an incident, but will not remember things previous to the event. This retrograde effect has been reported to span over some years before the brain trauma. However older memories are in some cases spared. Leading to the theory that consolidation over a long period of time will result in fully consolidated memories that are no longer dependent on the Papez circuit (in particular the hippocampus). [35] [36] Damage to different components of the Papez circuit is thought to lead to different forms of memory impairment (See table 1).
| Damaged Area | Resulting Effect |
|---|---|
| Hippocampal Formation | Bilateral damage causes anterograde and in some case retrograde amnesia [37] |
| Fornix | Can cause impairments of temporal order memory for retrograde information [38] |
| Mammillary Bodies | Korsakoff’s Syndrome; Anterograde Amnesia [39] |
| Mamillothalamic tract | Markedly impaired declarative memory (both anterograde and retrograde amnesia); linked with Korsakoff’s Syndrome [40] |
| Anterior Thalamic Nuclei | Linked to Korskaoff’s syndrome; spatial memory deficits. [41] |
| Cingulate Gyrus | Schizophrenia; disorientation as a major component of dementia [42] |
| Amygdala | Klüver-Bucy syndrome (visual agnosia) [43] |
| Hypothalamus | Anterograde and to a lesser extent retrograde amnesia; verbal memory impairment (Patient B.J) [44] |
| Prefrontal Cortex | Spatial memory deficit; Working memory deficits [45] |
| Association Cortex | Working memory deficits [46] |
Patient H.M. edit
In 1953, Patient H.M. underwent surgery to treat his epilepsy. The surgery consisted of bilateral removal of his medial temporal lobes (including the hippocampal formation and amygdala). As a direct result of this procedure H.M’s seizures decreased significantly, but more prominent was the severe anterograde amnesia that persisted. H.M showed impaired declarative memory functions for both forming new memories (anterograde amnesia) and retrieving old memories (retrograde amnesia). However the retrograde effect appeared to be on a time gradient, very old memories remained intact. Nondeclarative forms of memory appeared to be intact (i.e. priming tasks). From the hundreds of studies that H.M participated in, the general consensus implicates the medial temporal lobe as essential for the formation and temporal storage of memories, until fully consolidated. [47] [48]
Alzheimer’s edit
Alzheimer’s disease (AD) is a progressive neurodegenerative disease. Neuronal degeneration in AD begins in the medial temporal lobe structures, including the entorhinal cortex and hippocampus. Further neuroanotomic spread then incorporates other critical components of the Papez circuit, including the amygdala and anterior cingulate; which leads to the behavioural characteristics of AD. Eventually this spreads to involve the frontal regions, finally leading to death. AD is characterised by many symptoms, the most common being long-term memory loss, trouble with language, and confusion. [49]
Korsakoff’s Syndrome edit
Korsakoff’s syndrome is characterised by deficits in declarative memory, both anterograde and retrograde amnesia and confabulations and has also been linked with temporal order deficits. [50] Korsakoff’s syndrome is caused by damage to the medial thalamus and hypothalamus, in particular the mammillary bodies. [51] The most common cause of Korsakoff’s syndrome is a thiamine (vitamin B1) deficiency, through alcoholism or malnutrition. [52]
Klüver-Bucy Syndrome edit
Paul Bucy and Heinrich Klüver demonstrated that disrupting the Papez circuit by damage to the amygdala leads to profound effects on social and emotional behaviour. Klüver-Bucy syndrome is recognised by a combination of visual agnosia, placidity, hyperorality and hypersexuality. Visual agnosia is an inability to recognise objects or faces, and can lead to other memory disorders. [53]
References edit
- ^ Papez, J. W. (1937). A proposed mechanism of emotion. Archive of Neurological Psychiatry, 38(4), 725-733
- ^ Cannon, W. B. (1927). The James-Lange theory of emotion: A critical examination and an alternative theory. American Journal of Psychology, 39, 10-124.
- ^ Bard, P. (1934). The neuro-humoral basis of emotional reactions. In C. Murchinson (Ed.). A Handbook of General Experimental Psychology. Worcester: Clark University Press.
- ^ Dandy, W. E. (1931). Seat of consciousness. In L. Dean (Ed.). Practice of Surgery. Hagerston, Md: W. F. Prior Company Inc.
- ^ MacLean, P. D. (1949). Psychomatic disease and the ‘’visceral brain’’: Recent developments bearing on the Papez theory of emotion. Psychosomatic Medicine, 11, 338-353.
- ^ Maclean, P. D. (1952). Some psychiatric implications of physiological studies on frontotemporal portion of limbic system (visceral brain). Electroencephalography and Clinical Neurophysiology, 4(4), 407-418.
- ^ Papez, J. W. (1937). A proposed mechanism of emotion. Archive of Neurological Psychiatry, 38(4), 725-733
- ^ Martin, J. H. (2003). Limbic system and cerebral circuits for emotions, learning and memory. Neuroanatomy: text and atlas (3rd ed., pp.382). McGraw-Hill Companies.
- ^ Anderson, P., Morris, R., Amaral, D., Bliss, T., O’Keefe, J. (2007). Historical perspective: Proposed functions, biological characteristics and neurobiological models of the hippocampus. In J. Anderson, R. Morris, D Amaral et al. The Hippocampus Book (first ed., pp. 9-36). New York: Oxford University Press
- ^ Nieuwenhuys, R., Voogd. J., van Hujizen, C. (2008). The greater limbic system. The Human Central Nervous System,(4th ed., pp.917). Berlin/Heidelberg/New York: Springer-Verlag.
- ^ Wright, A. (1997). Limbic System: Hippocampus. In A. Wright (Eds.). Neuroscience Online. (1997). Retrieved from http://neuroscience.uth.tmc.edu/s4/chapter05.html
- ^ Aggleton, J. P. & Shaw, C. (1996). Amnesia and recognition memory: A re-analysis of psychometric data. Neuropsychologia, 34(1), 51-62.
- ^ Vann, S. D., & Aggleton, J. P. (2004). The mammillary bodies: Two memory systems in one? Nature Reviews Neuroscience, 5, 35-44.
- ^ Charles, R., Noback, D. A., Ruggiero, R. J., Demarest, Strominger, N. L. (2005). Thalamus. In Charles, R., Noback, D. A., Ruggiero, R. J., -Demarest, Strominger, N. L. (Ed.). The Human Nervous System: Structure and Function (pp.408-409).
- ^ Joseph. R. (2000). The Cingulate gyrus. In R. Joseph (Ed.) Neuropsychiatry, Neuropsychology, Clinical Neuroscience. (2000). (3rd ed). New York: Academic Press
- ^ Thomas, A. G., Koumellis, P & Dineen, R. A. (2011). The fornix in health and disease: An imaging review. RadioGraphics, 31, 1107-1121.
- ^ Yasuno, F., Hirati, M., Takimoto, H., Taniguchi, M., Nakagawa, Y., Ikekiri, Y … Takeda, M. (1999). Retrograde temporal order amnesia resulting from damage to the fornix. Journal of Neurology, Neurosurgery and Psychiatry, 67, 102-105.
- ^ Kwong, H. G., Hong, J. H., & Jang, S. H. (2011). Mammillothalamic tract in human brain: diffusion tensor tractography study. Neuroradiology, 53(8), 623-626
- ^ Gold, J, J., & Squire, L. R. (2006). The anatomy of amnesia: Neurohistological analysis of three new cases. Learning and Memory, 13, 699-710.
- ^ Maclean, P. D. (1952). Some psychiatric implications of physiological studies on frontotemporal portion of limbic system (visceral brain). Electroencephalography and Clinical Neurophysiology, 4(4), 407-418.
- ^ Maclean, P. D. (1952). Some psychiatric implications of physiological studies on frontotemporal portion of limbic system (visceral brain). Electroencephalography and Clinical Neurophysiology, 4(4), 407-418.
- ^ LeDoux, J. (2002). The emotional brain, fear and the amygdala. Cellular and Molecular Neurobiology, 23(4/5), 727-738.
- ^ Wright, A. (1997). Limbic System: Amygdala. In A. Wright (Eds.). Neuroscience Online. (1997). Retrieved from http://neuroscience.uth.tmc.edu/s4/chapter06.html
- ^ Wright, A. (1997). Hypothalamus: Structural Organization. In A. Wright (Eds.). Neuroscience Online. (1997). Retrieved from http://neuroscience.uth.tmc.edu/s4/chapter01.html
- ^ Yang, Y., & Raine, A. (2009). Prefrontal structural and functional brain imaging findings in antisocial, violent and psychopathic individuals: a meta-analysis. Psychiatry Research, 174(2), 81-88.
- ^ Klein, J. C., Rushworth, M. F. S, Behrens, T. E. J., Mackay, C. E., de Crespingny, A. J., D’Arceuil, H. & Johansen-Berg, H. (2010). Topography of connections between human prefrontal cortex and mediodorsal thalamus studied with diffusion tractography. Neuroimage, 51(2), 555-564.
- ^ Goldman-Rakic, P. S., Selemon, L. D., & Schwartz, M. L. (1984). Dual pathways connecting the dorsolateral prefrontal cortex with the hippocampal formation and parahippocampal cortex in the rhesus monkey. Neuroscience, 12(3), 719-743.
- ^ The association cortices. (2001). In D. Purves, G. J. Augustine., D. Fitzgerald., L. C. Katz., A. LaMantia., J. O McNamara & S. M. Williams. (Eds.). Neuroscience (2nd ed.)
- ^ Wright, A. (1997). Higher cortical functions: Association and executive processing. In A. Wright (Eds.). Neuroscience Online. (1997). Retrieved from http://neuroscience.uth.tmc.edu/s4/chapter09.html
- ^ Alvarez, P., & Squire, L. R., (1994) Memory consolidation and the medial temporal lobe, a simple network model. PNAS 91(15) 7041-7045
- ^ Tulving, E., & Markowitsch, H. J., (1998) Episodic and declarative memory: role of the hippocampus Hippocampus 8(3) 198-204
- ^ Tulving, E., (2002) Episodic Memory: from mind to brain, Annual Review of Psychology 53 1-25
- ^ Nadel, L., & Bohbot, V. (2001) Consolidation of Memory. Hippocampus 11(1) 56-60
- ^ Carlesimo, G.A., (2012) Memory Disorders in Patients with Cerebral Tumours Journal of Neuro-oncology 108(2) 253-256
- ^ MedicineNet(2012) Definition on Retrograde Amnesia. Retrived from: http://www.medterms.com/script/main/art.asp?articlekey=11959
- ^ Markowitsch, H. J., (2005) The neuroanatomy of memory. In Halligan, P.W., & Wade, T. D. (Eds.), Effectiveness of Rehabilitation for Cognitive Deficits 105-110. Oxford, England: Oxford University Press.
- ^ Zola-Morgan, S., Squire, L. R., & Amaral, D. G. (1989) Lesions of the hippocampal formation but not lesions of the fornix or the mammillary nuclei produce long-lasting memory impairment in monkeys. The Journal of Neuroscience 9 (3) 898-913
- ^ Yasuno, F., Hirati, M., Takimoto, H., Taniguchi, M., Nakagawa, Y., Ikekiri, Y … Takeda, M. (1999). Retrograde temporal order amnesia resulting from damage to the fornix. Journal of Neurology, Neurosurgery and Psychiatry, 67, 102-105.
- ^ Tanaka, Y., Miyazawa, Y., Akaoka, F., & Yamada, T. (1997) Amnesia Following Damage to the Mammillary Bodies Neurology 481) 160-165
- ^ Gold, J, J., & Squire, L. R. (2006) The anatomy of amnesia: Neurohistological analysis of three new cases. Learning and Memory 13 699-710
- ^ Harding, A., Halliday, G., Caine, D., & Kril, J. (1999) Degeneration of anterior thalamic nuclei differentiates alcoholics with amnesia. Brain 123(1) 141-154
- ^ Benes, F. M., Sorensen, I., Vincent, S. L., Bird, E. D., & Sathi, M. (1992) Increased Density of Glutamate-immunoreactive Vertical Processes in Superficial Lamina in Cingulaye Cortex of Schizophrenic Brain. Cerebral Cortex 2(6) 503-512
- ^ Hayman, L. A., Rexer, J. L., Pavol, M. A., Strite, D., & Meyers, C. A. (1998) Kluver-Bucy Syndrome After Bilateral Selective Damage of Amygdala and Its Cortical Connections The Journal of Neuropsychiatry and Clinical Neurosciences 10 354-358
- ^ Dusoir, H., Kapur, N., Byrnes, D. P., McKinstry, S., Hoare, R. D. (1989) Human Memory Disorder: Evidence from a penetrating paranasal brain injury. Brain 113(6) 1695-1706
- ^ McCarthy, G., Blamire, A. M., Puce, A., Nobre, A. C., Bloch, G., Hyder, F., … Shulman, R. G. (1994) Functional magnetic resonance imaging of human prefrontal cortex activation during a spatial working memory task. PNAS 91(18) 8690-8694
- ^ Colombo, M., D’Amato, M. R., Rodman, H. R., & Gross, C. G. (1990) Auditory Association Cortex Lesions Impair Audiory Short-Term Memory in Monkeys. Science 247 336-338
- ^ Corkin, S. (2002) What’s new with the amnesic patient H.M. Nature 3 153-160
- ^ Scoville, W. B., & Milner, B. (1957) Loss of recent memory after bilateral hippocampal lesions ‘’Journal of Neurology, Neurosurgery, Psychiatry’’ ‘’20’’(11) 11-21
- ^ Jicha, G. A., & Carr, S. A. (2010) Conceptual Evolution in Alzheimer’s disease: Implications for understanding the clinical phenotype of progressive neurodegenerative disease. Journal of Alzheimer’s Disease 19(1) 253-272
- ^ Shimamura, A. P., Janowsky, J. S., & Squire, L. R. (1990) Memory for the temporal order of events in patients with frontal love lesions and amnesic patients. Neuropsychologia 28(8) 803-813
- ^ Shimamura, A.P., Jernigan, T. L., & Squire, L. R. (1988) Korsakoff’s syndrome: radiology (CT) findings and neuropsychological correlates. Journal of Neuroscience 8(11) 4400-4410
- ^ Alzheimer’s Society (2012) What is Korsakoff’s Syndrome. Retrieved from http://www.alzheimers.org.uk/site/scripts/documents_info.php?documentID=98
- ^ Hooshmand, H., Sepdham, T., & Vries, J. K. (1974) Kluver-Bucy Syndrome: Successful Treatment with Carbamazepine. JAMA 229(13)
