 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ɛksˈtændi/ eks-TAN-dee |
| Trade names | Xtandi |
| Other names | MDV-3100; ASP-9785 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a612033 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth (capsules)[2][3] |
| Drug class | Nonsteroidal antiandrogen (NSAA)[4] |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | Rats: 89.7%[5] Humans: unknown (but at least 84.6% based on recovery from excretion)[6][3] |
| Protein binding | Enzalutamide: 97–98% (primarily to albumin)[2] NDME: 95%[2] |
| Metabolism | Liver (primarily CYP2C8 and CYP3A4)[2] |
| Metabolites | • NDME (active)[2][3] • Carboxylic acid derivative metabolite (inactive)[3] |
| Elimination half-life | Enzalutamide: 5.8 days (range 2.8–10.2 days)[2] NDME: 7.8–8.6 days[2] |
| Excretion | Urine: 71.0%[3] Bile: 13.6%[3] Feces: 0.39%[3] |
| Identifiers | |
| |
| Chemical and physical data | |
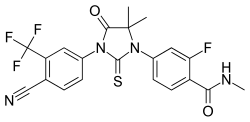
| Formula | C21H16F4N4O2S |
| Molar mass | 464.44 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Enzalutamide, sold under the brand name Xtandi, is a medication used to treat prostate cancer.[2] Specifically it is us to treat castration-resistant prostate cancer (mCRPC), and metastatic castration-sensitive prostate cancer (mCSPC).[2] It is taken by mouth.[2]
Common side effects include tiredness, back pain, diarrhea, joint pain, high blood pressure, and hot flashes.[2] Other side effects may include seizures, posterior reversible encephalopathy syndrome, allergic reactions, and falls.[2] Use during pregnancy, including by the male, may harm the baby.[2] There are a number of medication interactions.[2] It is a nonsteroidal antiandrogen (NSAA) which works by blocking androgens like testosterone, from binding to the androgen receptor.[4]
Enzalutamide was patented in 2006 and approved for medical use in the United States in 2012.[7][4] It is on the World Health Organization's List of Essential Medicines as an alternative abiraterone.[8] In the United Kingdom 4 weeks of treatment costs the NHS about £2,700 as of 2021.[9] This amount in the United States costs about 12,000 USD.[10]
References edit
- ^ a b "Enzalutamide (Xtandi) Use During Pregnancy". Drugs.com. 4 September 2018. Archived from the original on 22 December 2019. Retrieved 21 December 2019.
- ^ a b c d e f g h i j k l m n o p q "Xtandi- enzalutamide capsule". DailyMed. 9 July 2018. Archived from the original on 20 December 2015. Retrieved 21 December 2019.
- ^ a b c d e f g Gibbons JA, Ouatas T, Krauwinkel W, Ohtsu Y, van der Walt JS, Beddo V, de Vries M, Mordenti J (2015). "Clinical Pharmacokinetic Studies of Enzalutamide". Clin Pharmacokinet. 54 (10): 1043–55. doi:10.1007/s40262-015-0271-5. PMC 4580721. PMID 25917876.
- ^ a b c "Enzalutamide Monograph for Professionals". Drugs.com. Archived from the original on 22 December 2019. Retrieved 15 December 2021.
- ^ Kim TH, Jeong JW, Song JH, Lee KR, Ahn S, Ahn SH, Kim S, Koo TS (November 2015). "Pharmacokinetics of enzalutamide, an anti-prostate cancer drug, in rats". Archives of Pharmacal Research. 38 (11): 2076–82. doi:10.1007/s12272-015-0592-9. PMID 25956695. S2CID 26903608.
- ^ Benoist GE, Hendriks RJ, Mulders PF, Gerritsen WR, Somford DM, Schalken JA, van Oort IM, Burger DM, van Erp NP (2016). "Pharmacokinetic Aspects of the Two Novel Oral Drugs Used for Metastatic Castration-Resistant Prostate Cancer: Abiraterone Acetate and Enzalutamide". Clin Pharmacokinet. 55 (11): 1369–1380. doi:10.1007/s40262-016-0403-6. PMC 5069300. PMID 27106175.
- ^ Sawyers, Charles; Jung, Michael; Chen, Charlie; Ouk, Samedy; Welsbie, Derek; Tran, Chris; Wongvipat, John; Yoo, Dongwon (4 January 2007). "Diarylhydantoin compounds". Archived from the original on 5 October 2016. Retrieved 15 December 2021.
- ^ World Health Organization (2021). World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. hdl:10665/345533. WHO/MHP/HPS/EML/2021.02.
- ^ BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 991. ISBN 978-0857114105.
- ^ "Xtandi Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 17 December 2021. Retrieved 15 December 2021.