This article needs additional citations for verification. (December 2018) |
Stable nuclides are isotopes of a chemical element whose nucleons are in a configuration that does not permit them the surplus energy required to produce a radioactive emission. The nuclei of such isotopes are not radioactive and unlike radionuclides do not spontaneously undergo radioactive decay.[1] When these nuclides are referred to in relation to specific elements they are usually called that element's stable isotopes.
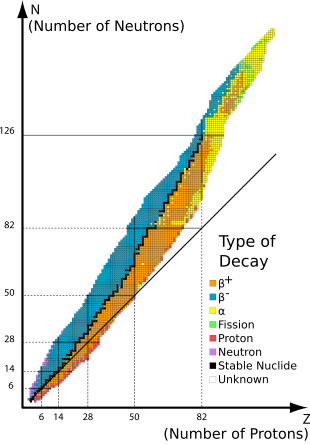
The 80 elements with one or more stable isotopes comprise a total of 251 nuclides that have not been shown to decay using current equipment. Of these 80 elements, 26 have only one stable isotope and are called monoisotopic. The other 56 have more than one stable isotope. Tin has ten stable isotopes, the largest number of any element.
Definition of stability, and naturally occurring nuclides
editMost naturally occurring nuclides are stable (about 251; see list at the end of this article), and about 35 more (total of 286) are known to be radioactive with long enough half-lives (also known) to occur primordially. If the half-life of a nuclide is comparable to, or greater than, the Earth's age (4.5 billion years), a significant amount will have survived since the formation of the Solar System, and then is said to be primordial. It will then contribute in that way to the natural isotopic composition of a chemical element. Primordial radioisotopes are easily detected with half-lives as short as 700 million years (e.g., 235U). This is the present limit of detection,[citation needed] as shorter-lived nuclides have not yet been detected undisputedly in nature except when recently produced, such as decay products or cosmic ray spallation.
Many naturally occurring radioisotopes (another 53 or so, for a total of about 339) exhibit still shorter half-lives than 700 million years, but they are made freshly, as daughter products of decay processes of primordial nuclides (for example, radium from uranium), or from ongoing energetic reactions, such as cosmogenic nuclides produced by present bombardment of Earth by cosmic rays (for example, 14C made from nitrogen).
Some isotopes that are classed as stable (i.e. no radioactivity has been observed for them) are predicted to have extremely long half-lives (sometimes 1018 years or more).[2] If the predicted half-life falls into an experimentally accessible range, such isotopes have a chance to move from the list of stable nuclides to the radioactive category, once their activity is observed. For example, 209Bi and 180W were formerly classed as stable, but were found to be alpha-active in 2003. However, such nuclides do not change their status as primordial when they are found to be radioactive.
Most stable isotopes on Earth are believed to have been formed in processes of nucleosynthesis, either in the Big Bang, or in generations of stars that preceded the formation of the Solar System. However, some stable isotopes also show abundance variations in the earth as a result of decay from long-lived radioactive nuclides. These decay-products are termed radiogenic isotopes, in order to distinguish them from the much larger group of 'non-radiogenic' isotopes.
Isotopes per element
editOf the known chemical elements, 80 elements have at least one stable nuclide. These comprise the first 82 elements from hydrogen to lead, with the two exceptions, technetium (element 43) and promethium (element 61), that do not have any stable nuclides. As of 2023, there were a total of 251 known "stable" nuclides. In this definition, "stable" means a nuclide that has never been observed to decay against the natural background. Thus, these elements have half-lives too long to be measured by any means, direct or indirect.
Stable isotopes:
- 1 element (tin) has 10 stable isotopes
- 5 elements have 7 stable isotopes apiece
- 7 elements have 6 stable isotopes apiece
- 11 elements have 5 stable isotopes apiece
- 9 elements have 4 stable isotopes apiece
- 5 elements have 3 stable isotopes apiece
- 16 elements have 2 stable isotopes apiece
- 26 elements have 1 single stable isotope.
These last 26 are thus called monoisotopic elements.[3] The mean number of stable isotopes for elements which have at least one stable isotope is 251/80 = 3.1375.
Physical magic numbers and odd and even proton and neutron count
editStability of isotopes is affected by the ratio of protons to neutrons, and also by presence of certain magic numbers of neutrons or protons which represent closed and filled quantum shells. These quantum shells correspond to a set of energy levels within the shell model of the nucleus; filled shells, such as the filled shell of 50 protons for tin, confers unusual stability on the nuclide. As in the case of tin, a magic number for Z, the atomic number, tends to increase the number of stable isotopes for the element.
Just as in the case of electrons, which have the lowest energy state when they occur in pairs in a given orbital, nucleons (both protons and neutrons) exhibit a lower energy state when their number is even, rather than odd. This stability tends to prevent beta decay (in two steps) of many even–even nuclides into another even–even nuclide of the same mass number but lower energy (and of course with two more protons and two fewer neutrons), because decay proceeding one step at a time would have to pass through an odd–odd nuclide of higher energy. Such nuclei thus instead undergo double beta decay (or are theorized to do so) with half-lives several orders of magnitude larger than the age of the universe. This makes for a larger number of stable even–even nuclides, which account for 150 of the 251 total. Stable even–even nuclides number as many as three isobars for some mass numbers, and up to seven isotopes for some atomic numbers.
Conversely, of the 251 known stable nuclides, only five have both an odd number of protons and odd number of neutrons: hydrogen-2 (deuterium), lithium-6, boron-10, nitrogen-14, and tantalum-180m. Also, only four naturally occurring, radioactive odd–odd nuclides have a half-life >109 years: potassium-40, vanadium-50, lanthanum-138, and lutetium-176. Odd–odd primordial nuclides are rare because most odd–odd nuclei beta-decay, because the decay products are even–even, and are therefore more strongly bound, due to nuclear pairing effects.[4]
Yet another effect of the instability of an odd number of either type of nucleon is that odd-numbered elements tend to have fewer stable isotopes. Of the 26 monoisotopic elements (those with only one stable isotope), all but one have an odd atomic number, and all but one has an even number of neutrons: the single exception to both rules is beryllium.
The end of the stable elements occurs after lead, largely because nuclei with 128 neutrons—two neutrons above the magic number 126—are extraordinarily unstable and almost immediately alpha-decay.[5] This contributes to the very short half-lives of astatine, radon, and francium. A similar phenomenon occurs to a much lesser extent with 84 neutrons—two neutrons above the magic number 82—where various isotopes of lanthanide elements alpha-decay.
Nuclear isomers, including a "stable" one
editThe 251 known stable nuclides include tantalum-180m, since even though its decay is automatically implied by its being "metastable", this has not been observed. All "stable" isotopes (stable by observation, not theory) are the ground states of nuclei, except for tantalum-180m, which is a nuclear isomer or excited state. The ground state, tantalum-180, is radioactive with half-life 8 hours; in contrast, the decay of the nuclear isomer is extremely strongly forbidden by spin-parity selection rules. It has been reported by direct observation that the half-life of 180mTa to gamma decay must be >1015 years. Other possible modes of 180mTa decay (beta decay, electron capture, and alpha decay) have also never been observed.
Still-unobserved decay
editIt is expected that improvement of experimental sensitivity will allow discovery of very mild radioactivity of some isotopes now considered stable. For example, in 2003 it was reported that bismuth-209 (the only primordial isotope of bismuth) is very mildly radioactive, with half-life (1.9 ± 0.2) × 1019 yr,[6][7] confirming earlier theoretical predictions[8] from nuclear physics that bismuth-209 would very slowly alpha decay.
Isotopes that are theoretically believed to be unstable but have not been observed to decay are termed observationally stable. Currently there are 105 "stable" isotopes which are theoretically unstable, 40 of which have been observed in detail with no sign of decay, the lightest in any case being 36Ar. Many "stable" nuclides are "metastable" in that they would release energy if they were to decay,[9] and are expected to undergo very rare kinds of radioactive decay, including double beta decay.
146 nuclides from 62 elements with atomic numbers from 1 (hydrogen) through 66 (dysprosium) except 43 (technetium), 61 (promethium), 62 (samarium), and 63 (europium) are theoretically stable to any kind of nuclear decay — except for the theoretical possibility of proton decay, which has never been observed despite extensive searches for it; and spontaneous fission (SF), which is theoretically possible for the nuclides with atomic mass numbers ≥ 93.[10]
Besides SF, other theoretical decay routes for heavier elements include:[10]
- alpha decay – 70 heavy nuclides (the lightest two are cerium-142 and neodymium-143)
- double beta decay – 55 nuclides
- beta decay – tantalum-180m
- electron capture – tellurium-123, tantalum-180m
- double electron capture
- isomeric transition – tantalum-180m
These include all nuclides of mass 165 and greater. Argon-36 is the lightest known "stable" nuclide which is theoretically unstable.[10]
The positivity of energy release in these processes means they are allowed kinematically (they do not violate conservation of energy) and, thus, in principle, can occur.[10] They are not observed due to strong but not absolute suppression, by spin-parity selection rules (for beta decays and isomeric transitions) or by the thickness of the potential barrier (for alpha and cluster decays and spontaneous fission).
Summary table for numbers of each class of nuclides
editThis is a summary table from List of nuclides. Note that numbers are not exact and may change slightly in the future, as nuclides are observed to be radioactive, or new half-lives are determined to some precision.
| Type of nuclide by stability class | Number of nuclides in class | Running total of nuclides in all classes to this point | Notes |
|---|---|---|---|
| Theoretically stable according to known decay modes, including alpha decay, beta decay, isomeric transition, and double beta decay | 146 | 146 | Contains the first 66 elements, except 43, 61, 62, and 63. If spontaneous fission is possible for the nuclides with mass numbers ≥ 93, then all such nuclides are unstable, so that only the first 40 elements would be stable; also, if protons decay, then there are no stable nuclides. |
| Energetically unstable to one or more known decay modes, but no decay yet seen. Considered stable until radioactivity confirmed. | 105[2][11] | 251 | Total is the observationally stable nuclides. All elements up to lead (except technetium and promethium) are included. |
| Radioactive primordial nuclides. | 35 | 286 | Includes bismuth, thorium, and uranium |
| Radioactive nonprimordial, but naturally occurring on Earth. | ~61 significant | ~347 significant | Cosmogenic nuclides from cosmic rays; daughters of radioactive primordials such as francium, etc. |
List of stable nuclides
editThe primordial radionuclides have been included for comparison; they are italicized and offset from the list of stable nuclides proper.
- Hydrogen-1
- Hydrogen-2
- Helium-3
- Helium-4
- no mass number 5
- Lithium-6
- Lithium-7
- no mass number 8
- Beryllium-9
- Boron-10
- Boron-11
- Carbon-12
- Carbon-13
- Nitrogen-14
- Nitrogen-15
- Oxygen-16
- Oxygen-17
- Oxygen-18
- Fluorine-19
- Neon-20
- Neon-21
- Neon-22
- Sodium-23
- Magnesium-24
- Magnesium-25
- Magnesium-26
- Aluminium-27
- Silicon-28
- Silicon-29
- Silicon-30
- Phosphorus-31
- Sulfur-32
- Sulfur-33
- Sulfur-34
- Sulfur-36
- Chlorine-35
- Chlorine-37
- Argon-36 (2E)
- Argon-38
- Argon-40
- Potassium-39
- Potassium-40 (B, E) – long-lived primordial radionuclide
- Potassium-41
- Calcium-40 (2E)*
- Calcium-42
- Calcium-43
- Calcium-44
- Calcium-46 (2B)*
- Calcium-48 (2B) – long-lived primordial radionuclide (B also predicted possible)
- Scandium-45
- Titanium-46
- Titanium-47
- Titanium-48
- Titanium-49
- Titanium-50
- Vanadium-50 (B, E) – long-lived primordial radionuclide
- Vanadium-51
- Chromium-50 (2E)*
- Chromium-52
- Chromium-53
- Chromium-54
- Manganese-55
- Iron-54 (2E)*
- Iron-56
- Iron-57
- Iron-58
- Cobalt-59
- Nickel-58 (2E)*
- Nickel-60
- Nickel-61
- Nickel-62
- Nickel-64
- Copper-63
- Copper-65
- Zinc-64 (2E)*
- Zinc-66
- Zinc-67
- Zinc-68
- Zinc-70 (2B)*
- Gallium-69
- Gallium-71
- Germanium-70
- Germanium-72
- Germanium-73
- Germanium-74
- Germanium-76 (2B) – long-lived primordial radionuclide
- Arsenic-75
- Selenium-74 (2E)
- Selenium-76
- Selenium-77
- Selenium-78
- Selenium-80 (2B)
- Selenium-82 (2B) – long-lived primordial radionuclide
- Bromine-79
- Bromine-81
- Krypton-78 (2E) – long-lived primordial radionuclide
- Krypton-80
- Krypton-82
- Krypton-83
- Krypton-84
- Krypton-86 (2B)
- Rubidium-85
- Rubidium-87 (B) – long-lived primordial radionuclide
- Strontium-84 (2E)*
- Strontium-86
- Strontium-87
- Strontium-88
- Yttrium-89
- Zirconium-90
- Zirconium-91
- Zirconium-92
- Zirconium-94 (2B)*
- Zirconium-96 (2B) – long-lived primordial radionuclide (B also predicted possible)
- Niobium-93
- Molybdenum-92 (2E)*
- Molybdenum-94
- Molybdenum-95
- Molybdenum-96
- Molybdenum-97
- Molybdenum-98 (2B)*
- Molybdenum-100 (2B) – long-lived primordial radionuclide
- Technetium – no stable isotopes
- Ruthenium-96 (2E)*
- Ruthenium-98
- Ruthenium-99
- Ruthenium-100
- Ruthenium-101
- Ruthenium-102
- Ruthenium-104 (2B)
- Rhodium-103
- Palladium-102 (2E)
- Palladium-104
- Palladium-105
- Palladium-106
- Palladium-108
- Palladium-110 (2B)*
- Silver-107
- Silver-109
- Cadmium-106 (2E)*
- Cadmium-108 (2E)*
- Cadmium-110
- Cadmium-111
- Cadmium-112
- Cadmium-113 (B) – long-lived primordial radionuclide
- Cadmium-114 (2B)*
- Cadmium-116 (2B) – long-lived primordial radionuclide
- Indium-113
- Indium-115 (B) – long-lived primordial radionuclide
- Tin-112 (2E)*
- Tin-114
- Tin-115
- Tin-116
- Tin-117
- Tin-118
- Tin-119
- Tin-120
- Tin-122 (2B)*
- Tin-124 (2B)*
- Antimony-121
- Antimony-123
- Tellurium-120 (2E)*
- Tellurium-122
- Tellurium-123 (E)*
- Tellurium-124
- Tellurium-125
- Tellurium-126
- Tellurium-128 (2B) – long-lived primordial radionuclide
- Tellurium-130 (2B) – long-lived primordial radionuclide
- Iodine-127
- Xenon-124 (2E) – long-lived primordial radionuclide
- Xenon-126 (2E)
- Xenon-128
- Xenon-129
- Xenon-130
- Xenon-131
- Xenon-132
- Xenon-134 (2B)*
- Xenon-136 (2B) – long-lived primordial radionuclide
- Caesium-133
- Barium-130 (2E) – long-lived primordial radionuclide
- Barium-132 (2E)*
- Barium-134
- Barium-135
- Barium-136
- Barium-137
- Barium-138
- Lanthanum-138 (B, E) – long-lived primordial radionuclide
- Lanthanum-139
- Cerium-136 (2E)*
- Cerium-138 (2E)*
- Cerium-140
- Cerium-142 (α, 2B)*
- Praseodymium-141
- Neodymium-142
- Neodymium-143 (α)
- Neodymium-144 (α) – long-lived primordial radionuclide
- Neodymium-145 (α)*
- Neodymium-146 (α, 2B)*
- no mass number 147§
- Neodymium-148 (α, 2B)*
- Neodymium-150 (2B) – long-lived primordial radionuclide
- Promethium - no stable isotopes
- Samarium-144 (2E)
- Samarium-146 (α) – probable long-lived primordial radionuclide
- Samarium-147 (α) – long-lived primordial radionuclide
- Samarium-148 (α) – long-lived primordial radionuclide
- Samarium-149 (α)*
- Samarium-150 (α)
- no mass number 151§
- Samarium-152 (α)
- Samarium-154 (2B)*
- Europium-151 (α) – long-lived primordial radionuclide
- Europium-153 (α)*
- Gadolinium-152 (α) – long-lived primordial radionuclide (2E also predicted possible)
- Gadolinium-154 (α)
- Gadolinium-155 (α)
- Gadolinium-156
- Gadolinium-157
- Gadolinium-158
- Gadolinium-160 (2B)*
- Terbium-159
- Dysprosium-156 (α, 2E)*
- Dysprosium-158 (α)
- Dysprosium-160 (α)
- Dysprosium-161 (α)
- Dysprosium-162 (α)
- Dysprosium-163
- Dysprosium-164
- Holmium-165 (α)
- Erbium-162 (α, 2E)*
- Erbium-164 (α, 2E)
- Erbium-166 (α)
- Erbium-167 (α)
- Erbium-168 (α)
- Erbium-170 (α, 2B)*
- Thulium-169 (α)
- Ytterbium-168 (α, 2E)*
- Ytterbium-170 (α)
- Ytterbium-171 (α)
- Ytterbium-172 (α)
- Ytterbium-173 (α)
- Ytterbium-174 (α)
- Ytterbium-176 (α, 2B)*
- Lutetium-175 (α)
- Lutetium-176 (B) – long-lived primordial radionuclide (α, E also predicted possible)
- Hafnium-174 (α) – long-lived primordial radionuclide (2E also predicted possible)
- Hafnium-176 (α)
- Hafnium-177 (α)
- Hafnium-178 (α)
- Hafnium-179 (α)
- Hafnium-180 (α)
- Tantalum-180m (α, B, E, IT)* ^
- Tantalum-181 (α)
- Tungsten-180 (α) – long-lived primordial radionuclide (2E also predicted possible)
- Tungsten-182 (α)*
- Tungsten-183 (α)*
- Tungsten-184 (α)*
- Tungsten-186 (α, 2B)*
- Rhenium-185 (α)
- Rhenium-187 (B) – long-lived primordial radionuclide (A also predicted possible)
- Osmium-184 (α) – long-lived primordial radionuclide (2E also predicted possible)
- Osmium-186 (α) – long-lived primordial radionuclide
- Osmium-187 (α)
- Osmium-188 (α)
- Osmium-189 (α)
- Osmium-190 (α)
- Osmium-192 (α, 2B)*
- Iridium-191 (α)
- Iridium-193 (α)
- Platinum-190 (α) – long-lived primordial radionuclide (2E also predicted possible)
- Platinum-192 (α)*
- Platinum-194 (α)
- Platinum-195 (α)*
- Platinum-196 (α)
- Platinum-198 (α, 2B)*
- Gold-197 (α)
- Mercury-196 (α, 2E)*
- Mercury-198 (α)
- Mercury-199 (α)
- Mercury-200 (α)
- Mercury-201 (α)
- Mercury-202 (α)
- Mercury-204 (2B)
- Thallium-203 (α)
- Thallium-205 (α)
- Lead-204 (α)*
- Lead-206 (α)*
- Lead-207 (α)*
- Lead-208 (α)*
- Bismuth ^^ and above –
- no stable isotopes
- no mass number 209 and above
- Bismuth-209 (α) – long-lived primordial radionuclide
- Thorium-232 (α, SF) – long-lived primordial radionuclide (2B also predicted possible)
- Uranium-235 (α, SF) – long-lived primordial radionuclide
- Uranium-238 (α, 2B, SF) – long-lived primordial radionuclide
- Plutonium-244 (α, SF) – probable long-lived primordial radionuclide (2B also predicted possible)
- Bismuth ^^ and above –
Abbreviations for predicted unobserved decay:[12][2][11]
α for alpha decay, B for beta decay, 2B for double beta decay, E for electron capture, 2E for double electron capture, IT for isomeric transition, SF for spontaneous fission, * for the nuclides whose half-lives have lower bound. Double beta decay has only been listed when beta decay is not also possible.
^ Tantalum-180m is a "metastable isotope", meaning it is an excited nuclear isomer of tantalum-180. See isotopes of tantalum. However, the half-life of this nuclear isomer is so long that it has never been observed to decay, and it thus is an "observationally stable" primordial nuclide, a rare isotope of tantalum. This is the only nuclear isomer with a half-life so long that it has never been observed to decay. It is thus included in this list.
^^ Bismuth-209 was long believed to be stable, due to its half-life of 2.01×1019 years, which is more than a billion times the age of the universe.
§ Europium-151 and samarium-147 are primordial nuclides with very long half-lives of 4.62×1018 years and 1.066×1011 years, respectively.
See also
edit- Isotope geochemistry
- List of elements by stability of isotopes
- List of nuclides (991 nuclides in order of stability, all with half-lives over one hour)
- Mononuclidic element
- Periodic table
- Primordial nuclide
- Radionuclide
- Stable isotope ratio
- Table of nuclides
- Valley of stability
References
edit- ^ "DOE explains ... Isotopes". Department of Energy, United States. Archived from the original on 14 April 2022. Retrieved 11 January 2023.
- ^ a b c Belli, P.; Bernabei, R.; Danevich, F. A.; et al. (2019). "Experimental searches for rare alpha and beta decays". European Physical Journal A. 55 (8): 140–1–140–7. arXiv:1908.11458. Bibcode:2019EPJA...55..140B. doi:10.1140/epja/i2019-12823-2. ISSN 1434-601X. S2CID 201664098.
- ^ Sonzogni, Alejandro. "Interactive Chart of Nuclides". National Nuclear Data Center: Brook haven National Laboratory. Archived from the original on 2018-10-10. Retrieved 2008-06-06.
- ^ Various (2002). Lide, David R. (ed.). Handbook of Chemistry & Physics (88th ed.). CRC. ISBN 978-0-8493-0486-6. OCLC 179976746. Archived from the original on 2017-07-24. Retrieved 2008-05-23.
- ^ Kelkar, N. G.; Nowakowski, M. (2016). "Signature of the N = 126 shell closure in dwell times of alpha-particle tunneling". Journal of Physics G: Nuclear and Particle Physics. 43 (105102). arXiv:1610.02069. Bibcode:2016JPhG...43j5102K. doi:10.1088/0954-3899/43/10/105102.
- ^ "WWW Table of Radioactive Isotopes". [permanent dead link]
- ^ Marcillac, Pierre de; Noël Coron; Gérard Dambier; Jacques Leblanc & Jean-Pierre Moalic (2003). "Experimental detection of α-particles from the radioactive decay of natural bismuth". Nature. 422 (6934): 876–878. Bibcode:2003Natur.422..876D. doi:10.1038/nature01541. PMID 12712201. S2CID 4415582.
- ^ de Carvalho H. G., de Araújo Penna M. (1972). "Alpha-activity of 209Bi". Lett. Nuovo Cimento. 3 (18): 720–722. doi:10.1007/BF02824346.
- ^ "NNDC – Atomic Masses". www.nndc.bnl.gov. Archived from the original on 2019-01-11. Retrieved 2009-01-17.
- ^ a b c d Nucleonica website
- ^ a b Tretyak, V.I.; Zdesenko, Yu.G. (2002). "Tables of Double Beta Decay Data — An Update". At. Data Nucl. Data Tables. 80 (1): 83–116. Bibcode:2002ADNDT..80...83T. doi:10.1006/adnd.2001.0873.
- ^ "Nucleonica :: Web driven nuclear science".
Book references
edit- Various (2002). Lide, David R. (ed.). Handbook of Chemistry & Physics (88th ed.). CRC. ISBN 978-0-8493-0486-6. OCLC 179976746. Archived from the original on 2017-07-24. Retrieved 2008-05-23.
External links
edit- The LIVEChart of Nuclides – IAEA
- AlphaDelta: Stable Isotope fractionation calculator
- National Isotope Development Center Reference information on isotopes, and coordination and management of isotope production, availability, and distribution
- Isotope Development & Production for Research and Applications (IDPRA) U.S. Department of Energy program for isotope production and production research and development
- Isosciences Archived 2021-01-18 at the Wayback Machine Use and development of stable isotope labels in synthetic and biological molecules