Hydralazine, sold under the brand name Apresoline among others, is a medication used to treat high blood pressure and heart failure.[2] This includes high blood pressure in pregnancy and very high blood pressure resulting in symptoms.[3] It has been found to be particularly useful in heart failure, together with isosorbide dinitrate, for treatment of people of African descent.[2] It is given by mouth or by injection into a vein.[3] Effects usually begin around 15 minutes and last up to six hours.[2]
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Apresoline, BiDil, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682246 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth, intravenous |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 26–50% |
| Protein binding | 85–90% |
| Metabolism | Liver |
| Onset of action | 5 to 30 min[2] |
| Elimination half-life | 2–8 hours, 7–16 hours (renal impairment) |
| Duration of action | 2 to 6 hrs[2] |
| Excretion | Urine |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.001.528 |
| Chemical and physical data | |
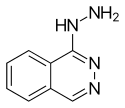
| Formula | C8H8N4 |
| Molar mass | 160.180 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Common side effects include headache and fast heart rate.[2] It is not recommended in people with coronary artery disease or in those with rheumatic heart disease that affects the mitral valve.[2] In those with kidney disease a low dose is recommended.[3] Hydralazine is in the vasodilator family of medications, so it is believed to work by causing the dilation of blood vessels.[2]
Hydralazine was discovered while scientists at Ciba were looking for a treatment for malaria.[4] It was patented in 1949.[5] It is on the World Health Organization's List of Essential Medicines.[6] In 2021, it was the 106th most commonly prescribed medication in the United States, with more than 6 million prescriptions.[7][8]
Medical use edit
Hydralazine is not used as a primary drug for treating hypertension because it elicits a reflex sympathetic stimulation of the heart (the baroreceptor reflex).[9] The sympathetic stimulation may increase heart rate and cardiac output, and in people with coronary artery disease may cause angina pectoris or myocardial infarction.[10] Hydralazine may also increase plasma renin concentration, resulting in fluid retention. To prevent these undesirable side effects, hydralazine is usually prescribed in combination with a beta blocker (e.g., propranolol) and a diuretic.[10] Beta-blockers licensed to treat heart failure in the UK include bisoprolol, carvedilol, and nebivolol.[11][12][13]
Hydralazine is used to treat severe hypertension, but is not a first-line therapy for essential hypertension. Hydralazine is often used to treat hypertension in pregnancy, though, with either labetalol and/or methyldopa.[14]
Hydralazine is commonly used in combination with isosorbide dinitrate for the treatment of congestive heart failure in black populations. This preparation, isosorbide dinitrate/hydralazine, was the first race-based prescription drug.[15]
It should not be used in people who have tachycardia, heart failure, constrictive pericarditis, lupus, a dissecting aortic aneurysm, or porphyria.[16]
Adverse effects edit
Prolonged treatment may cause a syndrome similar to lupus, which can become fatal if the symptoms are not noticed and drug treatment stopped.[16] Hydralazine is within the top three drugs that is known to induce systemic lupus and this adverse drug event is dose dependent yet significant.
Very common (>10% frequency) side effects include headache, tachycardia, and palpitations.[16]
Common (1–10% frequency) side effects include flushing, hypotension, anginal symptoms, aching or swelling joints, muscle aches, positive tests for atrial natriuretic peptide, stomach upset, diarrhea, nausea and vomiting, and swelling (sodium and water retention).[16]
Interactions edit
It may potentiate the antihypertensive effects of:[16]
Drugs subject to a strong first-pass effect such as beta blockers may increase the bioavailability of hydralazine.[16] The heart rate-accelerating effects of epinephrine (adrenaline) are increased by hydralazine, and coadministration may lead to toxicity.[16]
Mechanism of action edit
Hydralazine is a direct-acting smooth muscle relaxant and acts as a vasodilator primarily in resistance arterioles, also known as the smooth muscle of the arterial bed. The molecular mechanism involves inhibition of inositol trisphosphate-induced Ca2+ release from the sarcoplasmic reticulum in arterial smooth muscle cells.[17][18] By relaxing vascular smooth muscle, vasodilators act to decrease peripheral resistance, thereby lowering blood pressure and decreasing afterload.[10]
Metabolic products include the N-acetyl derivative, pyruvic acid hydrazone, and acetone hydrazone, each of which may also contribute to reducing blood pressure.[19]
Chemistry edit
Hydralazine belongs to the hydrazinophthalazine class of drugs.[20]
History edit
The antihypertensive activity of hydralazine was discovered by scientists at Ciba, who were trying to discover drugs to treat malaria; it was initially called C-5968 and 1-hydrazinophthalazine; Ciba's patent application was filed in 1945 and issued in 1949,[21][22][23] and the first scientific publications of its blood pressure-lowering activities appeared in 1950.[4][20][24] It was approved by the FDA in 1953.[25]
It was one of the first antihypertensive medications that could be taken by mouth.[9]
Research edit
Hydralazine has also been studied as a treatment for myelodysplastic syndrome in its capacity as a DNA methyltransferase inhibitor.[26]
References edit
- ^ "Prescription medicines: registration of new generic medicines and biosimilar medicines, 2017". Therapeutic Goods Administration (TGA). 21 June 2022. Retrieved 30 March 2024.
- ^ a b c d e f g h "Hydralazine Hydrochloride". The American Society of Health-System Pharmacists. Archived from the original on 21 December 2016. Retrieved 8 December 2016.
- ^ a b c World Health Organization (2009). Stuart MC, Kouimtzi M, Hill SR (eds.). WHO Model Formulary 2008. World Health Organization. p. 280. hdl:10665/44053. ISBN 9789241547659.
- ^ a b Wermuth CG (2 May 2011). The Practice of Medicinal Chemistry. Academic Press. p. 12. ISBN 9780080568775. Archived from the original on 26 February 2017.
- ^ Progress in Drug Research/Fortschritte der Arzneimittelforschung/Progrés des recherches pharmaceutiques. Birkhäuser. 2013. p. 206. ISBN 9783034870948. Archived from the original on 20 December 2016.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ "The Top 300 of 2021". ClinCalc. Archived from the original on 15 January 2024. Retrieved 14 January 2024.
- ^ "Hydralazine - Drug Usage Statistics". ClinCalc. Retrieved 14 January 2024.
- ^ a b Kandler MR, Mah GT, Tejani AM, Stabler SN, Salzwedel DM (November 2011). "Hydralazine for essential hypertension". The Cochrane Database of Systematic Reviews (11): CD004934. doi:10.1002/14651858.CD004934.pub4. PMID 22071816.
- ^ a b c Harvey RA, Harvey PA, Mycek MJ (2000). Lippincott's Illustrated Reviews: Pharmacology (2nd ed.). Philadelphia: Lippincott Williams & Wilkins. p. 190.
- ^ Joint Formulary Committee. "Bisoprolol fumarate". British National Formulary (online). London: BMJ Group and Pharmaceutical Press. Retrieved 13 March 2023.
- ^ Joint Formulary Committee. "Carvedilol". British National Formulary (online). London: BMJ Group and Pharmaceutical Press. Retrieved 13 March 2023.
- ^ Joint Formulary Committee. "Nebivolol". British National Formulary (online). London: BMJ Group and Pharmaceutical Press. Retrieved 13 March 2023.
- ^ Bhushan V, Lee TT, Ozturk A (2007). First Aid for the USMLE Step 1. New York: McGraw-Hill Medical. p. 251.
- ^ Ferdinand KC, Elkayam U, Mancini D, Ofili E, Piña I, Anand I, et al. (July 2014). "Use of isosorbide dinitrate and hydralazine in African-Americans with heart failure 9 years after the African-American Heart Failure Trial". The American Journal of Cardiology. 114 (1): 151–9. doi:10.1016/j.amjcard.2014.04.018. PMID 24846808.
- ^ a b c d e f g "Hydralazine Tablets 50mg". UK Electronic Medicines Compendium. 7 September 2016. Archived from the original on 27 February 2017.
- ^ Gurney AM, Allam M (January 1995). "Inhibition of calcium release from the sarcoplasmic reticulum of rabbit aorta by hydralazine". British Journal of Pharmacology. 114 (1): 238–244. doi:10.1111/j.1476-5381.1995.tb14931.x. PMC 1510175. PMID 7712024.
- ^ Ellershaw DC, Gurney AM (October 2001). "Mechanisms of hydralazine induced vasodilation in rabbit aorta and pulmonary artery". British Journal of Pharmacology. 134 (3): 621–631. doi:10.1038/sj.bjp.0704302. PMC 1572994. PMID 11588117.
- ^ Cohn JN, McInnes GT, Shepherd AM (2011). "Direct-acting vasodilators". Journal of Clinical Hypertension. 13 (9): 690–692. doi:10.1111/j.1751-7176.2011.00507.x. PMC 8108999. PMID 21896152.
- ^ a b Schroeder NA (January 1952). "The effect of 1-hydrasinophthalasine in hypertension". Circulation. 5 (1): 28–37. doi:10.1161/01.cir.5.1.28. PMID 14896450.
- ^ "Hydralazine". Drugbank. Archived from the original on 4 March 2017. Retrieved 4 March 2017.
- ^ "hydralazine". PubChem. Archived from the original on 4 March 2017. Retrieved 4 March 2017.
- ^ US2484029; see Example 1
- ^ Reubi FC (January 1950). "Renal hyperemia induced in man by a new phthalazine derivative". Proceedings of the Society for Experimental Biology and Medicine. 73 (1): 102–103. doi:10.3181/00379727-73-17591. PMID 15402536. S2CID 32603042.
- ^ "New Drug Application (NDA) 008303 Company: NOVARTIS Drug Name(s): Apresoline". FDA. Archived from the original on 26 February 2017. Retrieved 26 February 2017.
- ^ Singh V, Sharma P, Capalash N (May 2013). "DNA methyltransferase-1 inhibitors as epigenetic therapy for cancer". Current Cancer Drug Targets. 13 (4): 379–99. doi:10.2174/15680096113139990077. PMID 23517596.