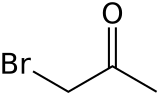
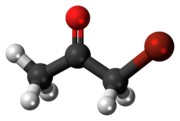
Bromoacetone is an organic compound with the formula CH3COCH2Br. It is a colorless liquid although impure samples appear yellow or even brown. It is a lachrymatory agent and a precursor to other organic compounds.
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
1-Bromopropan-2-one | |
| Other names
Bromoacetone
1-Bromo-2-propanone α-Bromoacetone Acetonyl bromide Acetyl methyl bromide Bromomethyl methyl ketone Monobromoacetone Martonite BA UN 1569 | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.009.027 |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C3H5BrO | |
| Molar mass | 136.976 g·mol−1 |
| Appearance | Colorless liquid |
| Density | 1.634 g/cm3 |
| Melting point | −36.5 °C (−33.7 °F; 236.7 K) |
| Boiling point | 137 °C (279 °F; 410 K) |
| Vapor pressure | 1.1 kPa (20 °C) |
| Hazards | |
| Flash point | 51.1 °C (124.0 °F; 324.2 K) |
| Safety data sheet (SDS) | MSDS at ILO |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Occurrence in nature
editBromoacetone is present (less than 1%) in the essential oil of a seaweed (Asparagopsis taxiformis) from the vicinity of the Hawaiian Islands.[2]
Synthesis
editBromoacetone is available commercially, sometimes stabilized with magnesium oxide. It was first described in the 19th century, attributed to N. Sokolowsky.[3]
Bromoacetone is prepared by combining bromine and acetone,[4] with catalytic acid. As with all ketones, acetone enolizes in the presence of acids or bases. The alpha carbon then undergoes electrophilic substitution with bromine. The main difficulty with this method is over-bromination, resulting in di- and tribrominated products. If a base is present, bromoform is obtained instead, by the haloform reaction.[5]
Applications
editIt was used in World War I as a chemical weapon, called BA by British and B-Stoff (Weisskreuz) by Germans. Due to its toxicity, it is not used as a riot control agent anymore. Bromoacetone is a versatile reagent in organic synthesis. It is, for example, the precursor to hydroxyacetone by reaction with aqueous sodium hydroxide.[6]
See also
editReferences
edit- ^ Merck Index, 11th Edition, 1389
- ^ Burreson, B. J.; Moore, R. E.; Roller, P. P. (1976). "Volatile halogen compounds in the alga Asparagopsis taxiformis (Rhodophyta)". Journal of Agricultural and Food Chemistry. 24 (4): 856–861. doi:10.1021/jf60206a040.
- ^ Wagner, G. (1876). "Sitzung der russischen chemischen Gesellschaft am 7./19. October 1876". Berichte der Deutschen Chemischen Gesellschaft. 9 (2): 1687–1688. doi:10.1002/cber.187600902196.
- ^ Levene, P. A. (1930). "Bromoacetone". Organic Syntheses. 10: 12; Collected Volumes, vol. 2, p. 88.
- ^ Reusch, W. (2013-05-05). "Carbonyl Reactivity". Virtual Textbook of Organic Chemistry. Michigan State University. Archived from the original on 2010-06-21. Retrieved 2007-10-27.
- ^ Levene, P. A.; Walti, A. (1930). "Acetol". Organic Syntheses. 10: 1; Collected Volumes, vol. 2, p. 5.