Amobarbital (formerly known as amylobarbitone or sodium amytal as the soluble sodium salt) is a drug that is a barbiturate derivative. It has sedative-hypnotic properties. It is a white crystalline powder with no odor and a slightly bitter taste. It was first synthesized in Germany in 1923. It is considered a short to intermediate acting barbiturate.
 | |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| License data |
|
| Routes of administration | By mouth, intramuscular, intravenous, rectal |
| Drug class | Barbiturate |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Hepatic |
| Elimination half-life | 8–42 hours |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.300 |
| Chemical and physical data | |
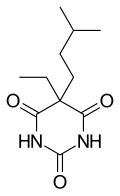
| Formula | C11H18N2O3 |
| Molar mass | 226.276 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
If amobarbital is taken for extended periods of time, physiological and psychological dependence can develop. Amobarbital withdrawal mimics delirium tremens and may be life-threatening. Amobarbital was manufactured by Eli Lilly and Company in the United States under the brand name Amytal in bright blue bullet shaped capsules (known as Pulvules) or pink tablets (known as Diskets)[2] containing 50, 100, or 200 milligrams of the drug. The drug was also manufactured generically.
Amobarbital was widely misused, known as "Blue Heavens" on the street. Amytal, as well as Tuinal, a combination drug containing equal quantities of secobarbital and amobarbital, were both manufactured by Eli Lilly until the late 1990s. However, as the popularity of benzodiazepines increased, prescriptions for these medications became increasingly rare beginning in the mid to late 1980s.
Pharmacology
editIn an in vitro study in rat thalamic slices, amobarbital worked by activating GABAA receptors, which decreased input resistance, depressed burst and tonic firing, especially in ventrobasal and intralaminar neurons, while at the same time increasing burst duration and mean conductance at individual chloride channels; this increased both the amplitude and decay time of inhibitory postsynaptic currents.[3]
Amobarbital has been used in a study to inhibit mitochondrial electron transport in the rat heart in an attempt to preserve mitochondrial function following reperfusion.[4]
A 1988 study found that amobarbital increases benzodiazepine receptor binding in vivo with less potency than secobarbital and pentobarbital (in descending order), but greater than phenobarbital and barbital (in descending order).[5] (Secobarbital > pentobarbital > amobarbital > phenobarbital > barbital)
It has an LD50 in mice of 212 mg/kg s.c.[citation needed]
Metabolism
editAmobarbital undergoes both hydroxylation to form 3'-hydroxyamobarbital,[6] and N-glucosidation[7] to form 1-(beta-D-glucopyranosyl)-amobarbital.[8]
Indications
editApproved
editUnapproved/off-label
editWhen given slowly by an intravenous route, sodium amobarbital has a reputation for acting as a so-called truth serum. Under the influence, a person will divulge information that under normal circumstances they would block. This was most likely due to loss of inhibition. As such, the drug was first employed clinically by William Bleckwenn at the University of Wisconsin to circumvent inhibitions in psychiatric patients.[9] The use of amobarbital as a truth serum has lost credibility due to the discovery that a subject can be coerced into having a "false memory" of the event.[10]
The drug may be used intravenously to interview patients with catatonic mutism, sometimes combined with caffeine to prevent sleep.[11]
It was used by the United States armed forces during World War II in an attempt to treat shell shock and return soldiers to the front-line duties.[12] This use has since been discontinued as the powerful sedation, cognitive impairment, and dis-coordination induced by the drug greatly reduced soldiers' usefulness in the field.
Contraindications
editThe following drugs should be avoided when taking amobarbital:
- Antiarrhythmics, such as verapamil and digoxin
- Antiepileptics, such as phenobarbital or carbamazepine
- Antihistamines, such as doxylamine and clemastine
- Antihypertensives, such as atenolol and propranolol
- Ethanol
- Benzodiazepines, such as diazepam, clonazepam, nitrazepam, alprazolam, or lorazepam
- Chloramphenicol
- Chlorpromazine
- Cyclophosphamide
- Ciclosporin
- Digitoxin
- Doxorubicin
- Doxycycline
- Methoxyflurane
- Metronidazole
- Narcotic analgesics, such as morphine and oxycodone
- Quinine
- Steroids, such as prednisone and cortisone
- Theophylline
- Warfarin
Interactions
editAmobarbital has been known to decrease the effects of hormonal birth control.[13]
Overdose
editSome side effects of overdose include confusion (severe); decrease in or loss of reflexes; drowsiness (severe); fever; irritability (continuing); low body temperature; poor judgment; shortness of breath or slow or troubled breathing; slow heartbeat; slurred speech; staggering; trouble in sleeping; unusual movements of the eyes; weakness (severe). Severe overdose may result in death without intervention.
Chemistry
editAmobarbital (5-ethyl-5-isoamylbarbituric acid), like all barbiturates, is synthesized by reacting malonic acid derivatives with urea derivatives. In particular, in order to make amobarbital, α-ethyl-α-isoamylmalonic ester is reacted with urea (in the presence of sodium ethoxide).[14][15]
Society and culture
editThe drug has been used to gain confessions from murderers such as Andres English-Howard, who strangled his girlfriend to death but claimed innocence. He was surreptitiously administered the drug by his lawyer, and under the influence of it he revealed why he strangled her and under what circumstances.[16]
On the night of August 28, 1951, the housekeeper of actor Robert Walker found him to be in an emotional state. She called Walker's psychiatrist who arrived and administered amobarbital for sedation. Walker was allegedly drinking prior to his emotional outburst, and it is believed the combination of amobarbital and alcohol resulted in a severe reaction. As a result, he passed out and stopped breathing, and all efforts to resuscitate him failed. Walker died at 32 years old.
The British actor and comedian Tony Hancock killed himself in Australia in 1968 using the drug in combination with alcohol.
Eli Lilly manufactured amobarbital under the brand name Amytal until it was discontinued in the 1980s and replaced largely by the benzodiazepine family of drugs. Amytal was also widely abused. Street names for amobarbital include "blues", "blue angels", "blue birds", "blue devils", and "blue heavens" due to their blue capsule.[17]
Cultural references
editIn Len Deighton's 1967 novel An Expensive Place to Die, a combination of amytal and LSD is used to make the unnamed protagonist respond to questioning about his activities.
In Thomas Pynchon's 1973 novel Gravity's Rainbow, sodium amytal is used by a military intelligence unit as some kind of truth serum to extract Tyrone Slothrop's (the novel's protagonist) ideas on racism of white Americans against Afro-Americans during the 1930s in his home state of Massachusetts (Chapter 1).
In the 1975 Columbo episode "A Deadly State of Mind" the victim Nadia Donner has Amobarbital found in her blood according to the autopsy report.[18][19][20]
In the 1994 comedic action thriller True Lies, the protagonist Harry Tasker (portrayed by Arnold Schwarzenegger) is given sodium amytal as a truth serum, when questioned by terrorists.
In 2001, the Law & Order: Special Victims Unit episode "Repression" (Season 3, Episode 1), a therapist (Shirley Knight) treats her patient with "sodium amytal". The character Dr. George Huang (BD Wong) claims that the drug leaves a patient so susceptible to suggestion, that the therapist is able to implant false memories in the patient.
In 2003, the animated series Sealab 2021 season 3, episode 7, "Tourist Season", Dr. Quentin Q. Quinn, voiced by Brett Butler, uses "sodium amytal" to induce amnesia in a group of tourists, in order to prevent them from taking action against Sealab as a result of a hare-brained scheme by Capt. Murphy. He gives the dose in a free alcoholic beverage as a parting gift before the tourists leave, and in quantities he describes that could "make an elephant forget".
In the 2005 movie, Hellraiser: Hellworld, the main antagonist uses "sodium amytal" to secretly anesthetize the people he believes were responsible for his son's death, and induce extreme hallucinations in them.
In 2016, television drama A Fist Within Four Walls episode 14, Man Zeun (portrayed by Princeton Lock) uses sodium amytal in an attempt to force gambling addict Lee Fat into paying debts.
In 2022, in "Diophantine Pseudonym", Episode 06 of Dan Brown's The Lost Symbol, sodium amytal is used by the CIA as a truth serum.
See also
editNotes
edit- ^ Anvisa (2023-03-31). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-04-04). Archived from the original on 2023-08-03. Retrieved 2023-08-16.
- ^ Winek, Charles L. (1965-02-01). "Dosage Form Names and Product Identification". American Journal of Health-System Pharmacy. 22 (2): 84. doi:10.1093/ajhp/22.2.82. ISSN 1079-2082.
- ^ Kim HS, Wan X, Mathers DA, Puil E (October 2004). "Selective GABA-receptor actions of amobarbital on thalamic neurons". British Journal of Pharmacology. 143 (4): 485–94. doi:10.1038/sj.bjp.0705974. PMC 1575418. PMID 15381635.
- ^ Stewart S, Lesnefsky EJ, Chen Q (May 2009). "Reversible blockade of electron transport with amobarbital at the onset of reperfusion attenuates cardiac injury". Translational Research. 153 (5): 224–31. doi:10.1016/j.trsl.2009.02.003. PMID 19375683.
- ^ Miller LG, Deutsch SI, Greenblatt DJ, Paul SM, Shader RI (1988). "Acute barbiturate administration increases benzodiazepine receptor binding in vivo". Psychopharmacology. 96 (3): 385–90. doi:10.1007/BF00216067. PMID 2906155. S2CID 29934652.
- ^ Maynert EW (October 1965). "The alcoholic metabolites of pentobarbital and amobarbital in man". The Journal of Pharmacology and Experimental Therapeutics. 150 (1): 118–21. PMID 5855308.
- ^ Tang BK, Kalow W, Grey AA (July 1978). "Amobarbital metabolism in man: N-glucoside formation". Research Communications in Chemical Pathology and Pharmacology. 21 (1): 45–53. PMID 684279.
- ^ Soine PJ, Soine WH (November 1987). "High-performance liquid chromatographic determination of the diastereomers of 1-(beta-D-glucopyranosyl)amobarbital in urine". Journal of Chromatography. 422: 309–14. doi:10.1016/0378-4347(87)80468-1. PMID 3437019.
- ^ Bleckwenn WJ (1930). "Sodium amytal in certain nervous and mental conditions". Wisconsin Medical Journal. 29: 693–696.
- ^ Stocks JT (September 1998). "Recovered memory therapy: a dubious practice technique". Social Work. 43 (5): 423–36. doi:10.1093/sw/43.5.423. PMID 9739631. (subscription required)
- ^ McCall WV (November 1992). "The addition of intravenous caffeine during an amobarbital interview". Journal of Psychiatry & Neuroscience. 17 (5): 195–7. PMC 1188455. PMID 1489761.
- ^ "Use of sodium amytal during WWII". Battle of the Bulge - program transcript. PBS. Archived from the original on 2009-03-14. Retrieved 2017-08-24.
Ben Kimmelman, Captain, 28th Infantry: The assumptions were that this would have some kind of cathartic effect, the sodium amytal, which the men called blue 88's. You know, the most effective artillery piece of the Germans was the 88 and this was blue 88s, because the sodium amytal was a blue tablet.
- ^ "Amobarbital | Memorial Sloan Kettering Cancer Center".
- ^ GB patent 191008, Layraud E, "The manufacture of unsymmetrical c.c.-dialkylbarbituric acids", issued 1923-10-25
- ^ US patent 1856792, Shonle HA, "Anhydrous alkali salts of 5,5-di-aliphatic-substituted barbituric acids and processes of producing them", issued 1932-05-03
- ^ Leung R (February 11, 2009). "Truth Serum: A Possible Weapon". 60 Minutes. CBS News. Archived from the original on April 5, 2005. Retrieved December 4, 2005.
- ^ "blue devils (amobarbital) - Memidex dictionary/thesaurus". www.memidex.com.
- ^ Hart, Harvey (1975-04-27), A Deadly State of Mind, Columbo, Peter Falk, George Hamilton, Lesley Ann Warren, retrieved 2024-01-11
- ^ A Deadly State of Mind | Echoes of Columbo, 16 September 2020, retrieved 2024-01-11
- ^ "Episode review: Columbo A Deadly State of Mind". THE COLUMBOPHILE BLOG. 2018-11-18. Retrieved 2024-01-11.