 | |
| Clinical data | |
|---|---|
| Trade names | Sivextro |
| Other names | TR-700, torezolid |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a614038 |
| Routes of administration | By mouth, intravenous |
| Drug class | Oxazolidinone antibiotic[1] |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 91% |
| Protein binding | 70–90% |
| Elimination half-life | 12 hours |
| Excretion | Feces |
| Identifiers | |
| |
| Chemical and physical data | |
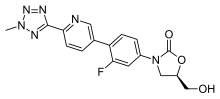
| Formula | C17H15FN6O3 |
| Molar mass | 370.344 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Tedizolid, sold under the brand name Sivextro, is an antibiotic used for skin and skin structure infection including cellulitis and skin abscesses.[3][1] This includes cases due to meticillin-resistant Staphylococcus aureus (MRSA).[1] It can be given by mouth or by gradual injection into a vein.[3]
Common side effects include nausea, headache, diarrhea, and vomiting.[1] Other side effects may include Clostridioides difficile infection.[3] Safety in pregnancy and breastfeeding is unclear.[3] It is in the oxazolidinone class of medications and works by blocking bacteria from making protein.[1]
Tedizolid was approved for medical use in the United States in 2014 and Canada and Europe in 2015.[1][3][4] It is on the World Health Organization's List of Essential Medicines as an alternative to linezolid.[5] In the United States a 6 day course of treatment costs about 2,300 USD as of 2021.[6] This amount in the United Kingdom costs the NHS about £862.[7]
References edit
- ^ a b c d e f g "Sivextro EPAR". European Medicines Agency (EMA). Archived from the original on 8 July 2020. Retrieved 5 July 2020. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ^ "Sivextro- tedizolid phosphate tablet, film coated Sivextro- tedizolid phosphate injection, powder, lyophilized, for solution". DailyMed. 22 June 2020. Archived from the original on 26 October 2020. Retrieved 24 October 2020.
- ^ a b c d e f "Tedizolid Monograph for Professionals". Drugs.com. Archived from the original on 25 January 2021. Retrieved 24 September 2021.
- ^ Canada, Health (4 May 2016). "Health Canada New Drug Authorizations: 2015 Highlights". www.canada.ca. Archived from the original on 20 February 2020. Retrieved 24 September 2021.
- ^ World Health Organization (2023). The selection and use of essential medicines 2023: web annex A: World Health Organization model list of essential medicines: 23rd list (2023). Geneva: World Health Organization. hdl:10665/371090. WHO/MHP/HPS/EML/2023.02.
- ^ "Tedizolid Prices, Coupons & Savings Tips - GoodRx". GoodRx. Archived from the original on 7 June 2016. Retrieved 24 September 2021.
- ^ BNF (80 ed.). BMJ Group and the Pharmaceutical Press. September 2020 – March 2021. p. 607. ISBN 978-0-85711-369-6.
{{cite book}}: CS1 maint: date format (link)