 | |
| Clinical data | |
|---|---|
| Pronunciation | /pæləˈnoʊsətrɒn/ pal-ə-NOH-sə-tron |
| Trade names | Aloxi |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a610002 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Intravenous, by mouth |
| Drug class | 5-HT3 antagonist[1] |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 97% (oral) |
| Protein binding | 62% |
| Metabolism | Liver, 50% (mostly CYP2D6-mediated, CYP3A4 and CYP1A2 also involved) |
| Elimination half-life | Approximately 40–50 hours |
| Excretion | Kidney, 80% (of which 49% unchanged); fecal (5 to 8%) |
| Identifiers | |
| |
| Chemical and physical data | |
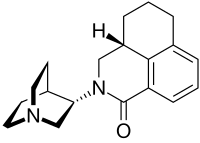
| Formula | C19H24N2O |
| Molar mass | 296.414 g·mol−1 |
| 3D model (JSmol) | |
| Specific rotation | [α]D −136° [α]D –94.1° (HCl) |
| Melting point | 87 to 88 °C (189 to 190 °F) |
| |
| |
| | |
Palonosetron, sold under the brand name Aloxi, is a medication used to prevent chemotherapy-induced nausea and vomiting (CINV) and postoperative nausea and vomiting.[2][3] In delayed CINV there is tentative data suggesting it may be more effective than granisetron.[4] It can be given by mouth or by injection into a vein.[1]
Common side effects include QT prolongation, slow heart rate, headache, constipation, and weakness.[3] Other side effects may include anaphylaxis and serotonin syndrome.[3] Use in pregnancy appears to be safe, but such use has not been well studied.[5] It is a 5-HT3 antagonist.[1]
Palonosetron was approved for medical use in the United States in 2003 and Europe in 2005.[3][2] It is on the World Health Organization's List of Essential Medicines as an alternative to ondansetron.[6] It is available as a generic medication.[1] In the United Kingdom it costs the NHS about £55 per dose as of 2021.[1] This amount in the United States costs about 50 USD.[7]
References
edit- ^ a b c d e f BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 456. ISBN 978-0857114105.
- ^ a b c "Aloxi". Archived from the original on 14 April 2021. Retrieved 25 October 2021.
- ^ a b c d e f "Palonosetron Monograph for Professionals". Drugs.com. Archived from the original on 21 January 2021. Retrieved 25 October 2021.
- ^ Billio A, Morello E, Clarke MJ (January 2010). Billio A (ed.). "Serotonin receptor antagonists for highly emetogenic chemotherapy in adults". The Cochrane Database of Systematic Reviews (1): CD006272. doi:10.1002/14651858.CD006272.pub2. PMID 20091591. (Retracted, see doi:10.1002/14651858.cd006272.pub3)
- ^ "Palonosetron (Aloxi) Use During Pregnancy". Drugs.com. Archived from the original on 25 November 2020. Retrieved 25 October 2021.
- ^ World Health Organization (2021). World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. hdl:10665/345533. WHO/MHP/HPS/EML/2021.02.
- ^ "Palonosetron Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 19 January 2021. Retrieved 25 October 2021.