 | |
| Clinical data | |
|---|---|
| Trade names | Dopram, Stimulex, Respiram, others |
| Other names | Doxapram hydrochloride |
| AHFS/Drugs.com | Monograph |
| Routes of administration | Intravenous |
| Drug class | Respiratory stimulant[1] |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Onset of action | 30 sec[2] |
| Duration of action | 5 to 12 min[2] |
| Identifiers | |
| |
| Chemical and physical data | |
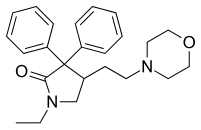
| Formula | C24H30N2O2 |
| Molar mass | 378.516 g·mol−1 |
| (verify) | |
Doxapram, sold under the brand name Dopram among others, is a medication used to treat respiratory depression after surgery or acute respiratory failure.[1] It is not; however, a preferred treatment.[2] It is given by injection into a vein.[1] Effects generally occur around 30 seconds and last for around 10 minutes.[2]
Common side effects include cough, shortness of breath, headache, dizziness, high blood pressure, flushing, sweating, vomiting, and muscle spasms.[2] Other side effects may include QT prolongation, seizure, and red blood cell breakdown.[2] While there is no evidence of harm in pregnancy, the manufacturer does not recommend.[1] It is a respiratory stimulant.[1]
Doxapram was approved for medical use in the United States in 1965.[2] It is available as a generic medication.[1] In the United Kingdom 5 vials of 100 mg costs the NHS about £130 as of 2021.[1] This amount in the United States is about 72 USD.[3]
References edit
- ^ a b c d e f g h BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 315. ISBN 978-0857114105.
- ^ a b c d e f g h "Doxapram Monograph for Professionals". Drugs.com. Archived from the original on 23 January 2021. Retrieved 27 December 2021.
- ^ "Dopram Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 27 January 2021. Retrieved 27 December 2021.