 | |||
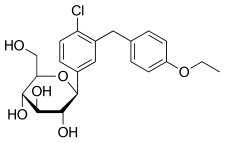 Haworth projection (bottom) | |||
| |||
| Clinical data | |||
|---|---|---|---|
| Pronunciation | /ˌdæpəɡlɪˈfloʊzɪn/ DAP-ə-glif-LOH-zin | ||
| Trade names | Forxiga, Farxiga, Edistride, others | ||
| Other names | BMS-512148; (1S)-1,5-anhydro-1-C-{4-chloro-3-[(4-ethoxyphenyl)methyl]phenyl}-D-glucitol | ||
| AHFS/Drugs.com | Monograph | ||
| License data |
| ||
| Pregnancy category |
| ||
| Routes of administration | By mouth (tablets) | ||
| Drug class | Sodium-glucose co-transporter 2 (SGLT2) inhibitor | ||
| Legal status | |||
| Legal status | |||
| Pharmacokinetic data | |||
| Bioavailability | 78% (after 10 mg dose) | ||
| Protein binding | ~91% | ||
| Metabolism | UGT1A9 (major), CYP (minor) | ||
| Metabolites | Dapagliflozin 3-O-glucuronide (inactive) | ||
| Elimination half-life | ~12.9 hours | ||
| Excretion | Urine (75%), feces (21%)[2] | ||
| Identifiers | |||
| |||
| Chemical and physical data | |||
| Formula | C21H25ClO6 | ||
| Molar mass | 408.88 g·mol−1 | ||
| 3D model (JSmol) | |||
| |||
| |||
| | |||
Dapagliflozin, sold under the brand name Farxiga among others, is a medication used to treat type 2 diabetes.[2] It is also used in heart failure with reduced ejection fraction.[3] In type 1 diabetes, it may be used together with insulin.[4] It is taken by mouth once a day.[2]
Common side effect include urinary tract infections, fungal infections of the groin, increased urination, nausea, and constipation.[2] Other side effects may include low blood sugar, especially when used with other diabetic medications, low blood pressure, Fournier gangrene, allergic reactions, and diabetic ketoacidosis.[2] Use is not recommended in pregnancy or breastfeeding.[4] It is of the gliflozin (SGLT2 inhibitor) class.[2]
Dapagliflozin was approved for medical use in the United States in 2014.[2] It was originally brought to market by AstraZeneca.[2] In the United States it costs about 500 USD per month as of 2020.[5] This amount costs the NHS in the United Kingdom about 40 pounds.[4] In 2017, it was the 259th most commonly prescribed medication in the United States, with more than one million prescriptions.[6][7]
References edit
- ^ a b "Dapagliflozin (Farxiga) Use During Pregnancy". Drugs.com. 30 August 2018. Retrieved 5 May 2020.
- ^ a b c d e f g h "Dapagliflozin Propanediol Monograph for Professionals". Drugs.com. Retrieved 3 December 2020.
- ^ "FDA approves new treatment for a type of heart failure". U.S. Food and Drug Administration (FDA) (Press release). 5 May 2020. Retrieved 5 May 2020. This article incorporates text from this source, which is in the public domain.
- ^ a b c BNF 79. London: Pharmaceutical Press. March 2020. p. 725. ISBN 978-0857113658.
- ^ "Dapagliflozin Prices, Coupons & Savings Tips". GoodRx. Retrieved 3 December 2020.
- ^ "The Top 300 of 2020". ClinCalc. Retrieved 11 April 2020.
- ^ "Dapagliflozin - Drug Usage Statistics". ClinCalc. Retrieved 11 April 2020.
