 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Many[1] |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Intramuscular[2] |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| Chemical and physical data | |
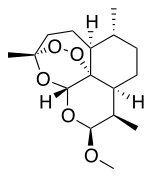
| Formula | C16H26O5 |
| Molar mass | 298.374 g/mol g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 86 to 88 °C (187 to 190 °F) |
| |
| |
| | |
Artemether is a medication used for the treatment of malaria.[2] The injectable form is specifically used for severe malaria rather than quinine.[2] In adults, it may not be as effective as artesunate.[2] It is given by injection in a muscle.[2] It is also available by mouth in combination with lumefantrine, known as artemether/lumefantrine.[4][5]
Artemether causes relatively few side effects.[6] An irregular heartbeat may rarely occur.[6] While there is evidence that use during pregnancy may be harmful in animals, there is no evidence of concern in humans.[6] The World Health Organization (WHO) therefore recommends its use during pregnancy.[6] It is in the artemisinin class of medication.[6]
Artemether has been studied since at least 1981, and been in medical use since 1987.[7] It is on the World Health Organization's List of Essential Medicines.[8] The wholesale cost in the developing world is between US$0.38 and US$16.47 per vial.[9] The combination form costs between US$100 and US$200 for a course of treatment in the United States.[10]
References
edit- ^ "Artemether - Drugs.com". www.drugs.com. Archived from the original on 20 December 2016. Retrieved 7 December 2016.
- ^ a b c d e Esu, Ekpereonne B.; Effa, Emmanuel E.; Opie, Oko N.; Meremikwu, Martin M. (18 June 2019). "Artemether for severe malaria". The Cochrane Database of Systematic Reviews. 6: CD010678. doi:10.1002/14651858.CD010678.pub3. ISSN 1469-493X. PMC 6580442. PMID 31210357.
- ^ Cite error: The named reference
whowas invoked but never defined (see the help page). - ^ "Artemether and Lumefantrine". The American Society of Health-System Pharmacists. Archived from the original on 20 December 2016. Retrieved 28 November 2016.
- ^ "Coartem- artemether and lumefantrine tablet". DailyMed. 5 August 2019. Archived from the original on 26 July 2020. Retrieved 26 April 2020.
- ^ a b c d e Kovacs, SD; Rijken, MJ; Stergachis, A (February 2015). "Treating severe malaria in pregnancy: a review of the evidence". Drug Safety. 38 (2): 165–81. doi:10.1007/s40264-014-0261-9. PMC 4328128. PMID 25556421.
- ^ Rao, Yi; Zhang, Daqing; Li, Runhong (2016). Tu Youyou and the Discovery of Artemisinin: 2015 Nobel Laureate in Physiology or Medicine. World Scientific. p. 162. ISBN 9789813109919. Archived from the original on 2017-09-10.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ "Artemether". International Drug Price Indicator Guide. Archived from the original on 22 January 2018. Retrieved 1 January 2016.
- ^ Hamilton, Richart (2015). Tarascon Pocket Pharmacopoeia 2015 Deluxe Lab-Coat Edition. Jones & Bartlett Learning. p. 45. ISBN 9781284057560.