 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Eliquis, others |
| Other names | BMS-562247-01 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a613032 |
| License data | |
| Routes of administration | By mouth |
| Drug class | DOAC (direct factor Xa inhibitor)[1] |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | ~50% |
| Protein binding | ~87% |
| Metabolism | CYP3A4, CYP3A5, CYP1A2 and others |
| Elimination half-life | 9–14 h |
| Excretion | Bile (75%), kidney (25%) |
| Identifiers | |
| |
| Chemical and physical data | |
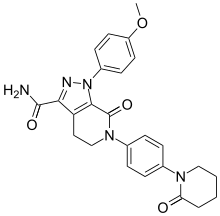
| Formula | C25H25N5O4 |
| Molar mass | 459.497 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Apixaban, sold under the brand name Eliquis among others, is an anticoagulant medication used to treat and prevent blood clots and to prevent stroke in people with nonvalvular atrial fibrillation (AF).[1][2][4] Specifically it is used to prevent blood clots following hip or knee replacement and in those with a history of prior clots.[1][4] It is used as an alternative to warfarin and does not require monitoring by blood tests.[1] It is taken by mouth.[1]
Common side effects include bleeding and nausea.[1][2] Other side effects may include bleeding around the spine and allergic reactions.[1] Use is not recommended during pregnancy or breastfeeding.[2] Use appears to be relatively safe in those with mild kidney problems.[2] Compared to warfarin it has fewer interactions with other medications.[5] It is a direct factor Xa inhibitor.[1]
Apixaban was approved for medical use in the European Union in May 2011 and in the United States in December 2012.[6][7][1] A month supply in the United Kingdom costs the NHS about £53 as of 2020.[2] In the United States the wholesale cost of this amount is about $427.[8] In 2017, it was the 93rd most commonly prescribed medication in the United States with more than eight million prescriptions.[9][10] In December 2019, generic versions were approved in the United States.[4]
References edit
- ^ a b c d e f g h i j k "Apixaban Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Archived from the original on 27 March 2019. Retrieved 27 March 2019.
- ^ a b c d e f g BNF (80 ed.). BMJ Group and the Pharmaceutical Press. September 2020 – March 2021. p. 133-134. ISBN 978-0-85711-369-6.
- ^ "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 20 September 2020. Retrieved 9 September 2020.
- ^ a b c "FDA approves first generics of Eliquis". U.S. Food and Drug Administration (FDA). 23 December 2019. Archived from the original on 23 December 2019. Retrieved 23 December 2019. This article incorporates text from this source, which is in the public domain.
- ^ Kiser, Kathryn (2017). Oral Anticoagulation Therapy: Cases and Clinical Correlation. Springer. p. 11. ISBN 9783319546438. Archived from the original on 27 March 2019. Retrieved 27 March 2019.
- ^ "Eliquis EPAR". European Medicines Agency. Archived from the original on 11 August 2020. Retrieved 22 April 2020. This article incorporates text from this source, which is in the public domain.
- ^ "Drug Approval Package: Eliquis (apixaban) NDA #202155". U.S. Food and Drug Administration (FDA). 13 February 2013. Archived from the original on 23 December 2019. Retrieved 23 December 2019.
- ^ "NADAC as of 2019-02-27". Centers for Medicare and Medicaid Services. Archived from the original on 6 March 2019. Retrieved 3 March 2019.
- ^ "The Top 300 of 2020". ClinCalc. Archived from the original on 18 March 2020. Retrieved 11 April 2020.
- ^ "Apixaban - Drug Usage Statistics". ClinCalc. Archived from the original on 28 February 2020. Retrieved 11 April 2020.