| Endovascular aneurysm repair | |
|---|---|
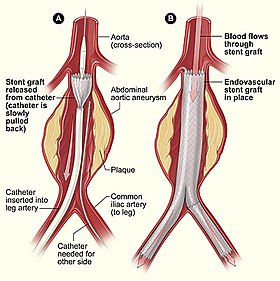 Endovascular aneurysm repair | |
| ICD-9-CM | 39.51, 39.52, 39.7 |
Endovascular aneurysm repair (or endovascular aortic repair) (EVAR) is a type of endovascular surgery used to treat pathology of the aorta, most commonly an abdominal aortic aneurysm (AAA) or thoracic aortic aneurysm, the procedure then specifically termed TEVAR (thoracic endovascular aortic/aneurysm repair). The procedure involves the placement of an expandable stent graft within the aorta to treat aortic disease without operating directly on the aorta.
Has replaced user:bakerstmd/Open aortic surgery as the predominant method of repairing AAA in the US.
Uses
editStandard EVAR is appropriate for aneurysms that begin below the renal arteries, where there exists an adequate length of normal aorta (the "proximal aortic neck") for reliable attachment of the endograft without leakage of blood around the device ("endoleak").
Patients with aneurysms require elective repair of their aneurysm when it reaches a diameter large enough (typically greater than 5.5 cm) where the risk of rupture is greater than the risk of surgery. Repair is also warranted for aneurysms that rapidly enlarge or those that have been the source of emboli (debris from the aneurysm that dislodge and travel into other arteries). Lastly, repair is also indicated for aneurysms that are the source of pain and tenderness, which may indicate impending rupture. The options for repair include traditional open surgery or endovascular repair.
EVAR is also used for cases of aortic rupture.
Use in Aortic Dissection
editEndografts have been used in patients with aortic dissection, noting the extremely complex nature of open surgical repair in these patients. While the results have been excellent, no significant survival advantage has been demonstrable compared with open surgical repair. In uncomplicated aortic dissections, no benefit has been demonstrated over medical management alone. In uncomplicated type B aortic dissection, TEVAR does not seem either improve or compromise 2-year survival and adverse event rates.[1] Its use in complicated aortic dissection is under investigation.
Patient Screening
editBefore patients are deemed to be a suitable candidate for this treatment, they have to go through a rigorous set of tests. These include a CT scan of the complete thorax/abdomen/pelvis, and blood tests. The CT scan gives precise measurements of the aneurysm and the surrounding anatomy. In particular the calibre/tortuosity of the iliac arteries and the relationship of the neck of the aneurysm to the renal arteries are important determinants of whether the aneurysm is amenable to endoluminal repair. In certain occasions that the renal arteries are too close to the aneurysm, the custom-made fenestrated graft stent is now an accepted alternative to doing open surgery.
Contra-indications
editA patients anatomy can be unsuitable for EVAR in several ways. Most commonly, in an infrarenal aneurysm, a potential EVAR candidate lacks adequate length of normal-diameter aorta between the aneurysm and the takeoff of the renal arteries, the "infra-renal neck". Other relative contra-indications include prohibitively small iliac arteries, aneurysmal iliac arteries, prohibitively small femoral arteries, or circumferential calcification of the femoral or iliac arteries.
In addition to a short proximal aortic neck, the neck may be angulated, large in diameter, or shaped like a funnel (conical) where the neck diameter at the top is larger than the neck diameter at the bottom. Along with a short proximal aortic neck, necks with any of these characteristics are called “hostile necks” and endovascular repair can be either contraindicated or associated with early late complications of endoleak, or endograft migration, or both.
May of the advances in EVAR technique aim to adapt EVAR for these situations, and advanced techniques allow EVAR to be employed in patients who previously were not candidates.
Technique
editThe procedure is carried out in a sterile environment under x-ray fluoroscopic guidance. It is usually carried out by a vascular surgeon, and occasionally by an interventional radiologist, general surgeon or interventional cardiologist. The procedure can be performed under general, regional (spinal or epidural) or even local anesthesia.
Access to the patient's femoral arteries can be through small incisions at the top of each leg ("the groins"), or percutaneously through a needle and sheath. In either case, vascular sheaths are introduced into the patient's femoral arteries, through which the guidewires, catheters and eventually the endograft is passed.
Diagnostic angiography images or 'runs' are captured of the aorta to determine the location of the patient's renal arteries, so the stent graft can be deployed without blocking these. Failure to achieve this will cause renal failure. Precision and control of endograft deployment is extremely important. The "main" body of the endograft is placed first, followed by the "limbs" which join the main body and extend to the iliac arteries, effectively bypassing the aneurysm sac from blood flow.
The abdominal aneurysm extends down to the common iliac arteries in about 25%-30% of patients. In such cases, the iliac limbs can be extended into the external iliac artery to bypass a common iliac aneurysm. Alternatively, a specially designed endograft, (iliac branch device) can be used. The preservation of the hypogastric (internal iliac) arteries is important to prevent buttock claudication and impotence, and every effort should be made to preserve flow to at least one hypogastric artery.
The endograft, once in place, acts as an artificial lumen for blood to flow through, and not into the surrounding aneurysm sac. This reduces the pressure in the aneurysm, which itself will usually thrombose and shrink in size over time.[2]
Staging such procedures is common. One example is revascularization of the left common carotid artery and/or the left subclavian artery from the innominate artery or the right common carotid artery to allow treatment of a thoracic aortic aneurysm that encroaches proximally into the aortic arch. These "extra-anatomic bypasses" can be performed without an invasive thoracotomy. Continued design improvement in stent graft including branched endografts will reduce but not eliminate this category of surgery.
Endovascular procedures aim to reduce the morbidity and mortality of treating arterial disease in a patient population that is increasingly older and less fit than when major open repairs were developed and popularized. Even in the early days, significant risks were accepted in the understanding that the large open operation was the only option. That is not the case in most patients today.
Studies that assign aneurysm patients to treatment with EVAR or traditional open surgery have uniformly demonstrated fewer early complications with the minimally-invasive approach. Some studies have also observed a lower mortality rate with EVAR. The reduction in death, however, does not persist long-term. After a few years the survival after repair is similar with EVAR or open surgery. This observation may be the result of durability problems with early endografts, with a corresponding need for additional procedures to repair endoleaks and other device-related issues. Newer, improved technology may reduce the need for such secondary procedures. If so, the results of EVAR may improve to the point where long-term survival benefit becomes evident.
The complications of EVAR can be divided into those that are related to the repair procedure and those related to the endograft device. For example, a myocardial infarction that occurs immediately after the repair is normally related to the procedure and not the device. By contrast, the development of an endoleak from degeneration of endograft fabric would be a device-related complication.
Percutaneous EVAR
editStandard EVAR involves a surgical cut-down on either the femoral or iliac arteries, with the creation of a 4-6cm incision. Like many surgical procedures, EVAR has advanced to a more minimally invasive technique, by accessing the femoral arteries "percutaneously". In percutaneous EVAR (PEVAR), small, sub-centimeter incisions are made over the femoral artery, and endovascular techniques are used to place the device over a wire.
Fenestrated EVAR
editIn certain occasions, a specially designed custom-made graft device ("endograft"), which has holes (fenestrations) on the graft body to maintain the patency of the visceral arteries, is used for the procedure, which is called FEVAR (fenestrated endovascular aortic/aneurysm repair). When the aneurysm begins at close to the renal arteries, standard EVAR may be contraindicated since there will be an inadequate length of suitable aorta for the endograft attachment. In these cases a fenestrated endograft may be useful, where the attachment of the endograft to the aorta may be placed above the renal arteries with each fenestration opposite a renal artery so that blood flow to the kidneys is maintained.
Branched EVAR
editThoraco-abdominal aortic aneurysms (TAAA) involve the aorta in the chest and abdomen. As such, major branch arteries to the head, arms, spinal cord, intestines and kidneys may originate from the aneurysm. An endovascular repair of a TAAA is only possible if blood flow to these critical arteries is preserved. Hybrid procedures offer one option, but a more direct approach involves the use of a branched endograft. Dr. Timothy Chuter pioneered this approach, with a completely endovascular solution. After partial deployment of the main body of an endograft, separate endograft limbs are deployed from the main body to each major aortic branch. This procedure is long, technically difficult, and currently only performed in a few centers. When the aneurysm begins above the renal arteries, neither fenestrated endografts nor EndoAnchoring of an infrarenal endograft are useful (an open surgical repair may be necessary). Alternatively, a "branched" endograft may be used. A branched endograft has graft limbs that branch off of the main portion of the device to directly provide blood flow to the kidneys or the visceral arteries.
Hybrid Procedures
editOn occasion there is inadequate length or quality of the proximal or distal aortic neck. In these cases, a fully minimally invasive option is not possible. One solution, however, is a hybrid repair, which combines an open surgical bypass with EVAR or TEVAR. In hybrid procedures, the endograft is positioned over major aortic branches. While such a position would normally cause problems from disruption of blood flow to the covered branches (renal, visceral, or branches to the head or arms), the prior placement of bypass grafts to these critical vessels allowed deployment of the endograft at a level that would otherwise not be possible.
Adjunctive Procedures in EVAR
edit- Snorkel: A covered stent placed into a visceral vessel adjacent to the main body of the EVAR device. The aortic lumen of the visceral stent is directed superiorly, resembling a snorkel.
- Chimney: In TEVAR, a covered stent placed from the ascending aorta to a great vessel (e.g. innominate artery) and adjacent to the main body of the EVAR is termed a chimney. In anatomic position, blood flows superiorly through a chimney-stent-graft into the great vessel, just as smoke flows up a chimney.
- Periscope: Like a snorkel, a periscope stent-graft provides flow to a visceral vessel, but in retrograde fashion, with the aortic lumen inferior to the main body of the EVAR device.
- Stents: Large (Palmaz) stents has been used to treat proximal endoleaks, as have aortic extension cuffs to treat endograft migration.
- Coils and glues have been used to treat type I endoleaks, with inconsistent success.[3]
- EndoAnchors:EndoAnchors are another option in patients with “hostile necks” that are short, large in diameter, angulated, or conical. EndoAnchors, unlike large stents, coils, and glues, are cleared for marketing in patients with type I endoleaks. EndoAnchors are small, helically-shaped devices that are screwed through the endograft and into the aortic wall. After placement of multiple EndoAnchors, the configuration resembles, in principle, a traditional hand-sewn connection ("surgical anastomosis"). EndoAnchors increase the strength of the endograft-to-aortic connection and improve sealing to reduce the risk of blood flow around the endograft (type Ia endoleak).[4] EndoAnchors have been used successfully to treat endoleaks and, in concert with an aortic extension endograft, to treat migration of the original endograft.[5][6][7][8][9] EndoAnchors have been used prophylactically in patients with a hostile neck.[10] Initial results have been promising but long-term outcome is not yet available.
Risks/Complications
editDurability and problems such as 'endoleaks' may require careful surveillance and adjuvant procedures to ensure success of the EVAR or EVAR/hybrid procedure. CT Angiography (CTA) imaging has in particular made a key contribution to planning, success, durability in this complex area of vascular surgery.
Procedure related
editArterial dissection,contrast-induced renal failure, thromboembolizaton, ischemic colitis, groin hematoma, wound infection, type II endoleaks, myocardial infarction, congestive heart failure, cardiac arrhythmias, respiratory failure.
Device related
editEndograft migration, aneurysm rupture, graft limb stenosis/kinking, type I/III/IV endoleaks
Endoleaks
editAn endoleak is a leak into the aneurysm sac after endovascular repair. Five types of endoleaks exist:[2]
- Type I - Perigraft leakage at proximal or distal graft attachment sites (near the renal and iliac arteries)
- Type II - Retrograde flow from collateral branches such as the lumbar and inferior mesenteric arteries
- Type III - Leakage between overlapping parts of the stent (i.e. connection between overlapping components) or rupture through graft material.
- Type IV - Leakage through the graft wall due to the quality (porosity) of the graft material
- Type V - Expansion of the aneurysm sac without an identifiable leak. Also called "endotension".
Recovery after EVAR
editUnlike traditional aortic repair, standard recovery after EVAR is remarkably straightforward. Patients who have undergone EVAR typically spend one night in the hospital to be monitored, although it has been suggested that EVAR can be performed as a same-day procedure. [11]
Patients are advised to slowly return to normal activity. There are no specific activity restrictions after EVAR, however patients typically are seen by their surgeon within one month after EVAR to begin post-EVAR surveillance.
History
editNoting the morbidity of open surgical repair of aortic aneurysms, particularly in the elderly and medically fragile patient, Dr. Juan Parodi began work on a less invasive procedure in 1976 at the Cleveland Clinic.[12] A few years later, Nicholas Volodos began similar work in Kharkov, Soviet Union and introduced in an article written in 1988.[13] Volodos performed the first endovascular repair of a thoracic aneurysm in 1987: a false aneurysm that likely developed from a motor vehicle accident three decades before. This patient did well, with successful endovascular thoracic aneurysm repair and died of unrelated causes 18 years later.
Parodi performed the first successful endovascular repair of an abdominal aortic aneurysm in 1990, although Volodos had attempted EVAR one year before in an unsuccessful procedure that was complicated by twisting an occlusion of the graft, requiring immediate conversion to traditional open surgical aneurysm repair.
The modern endovascular device used to repair Abdominal aortic aneurysm, which is bifurcated and modular, was pioneered and first employed by Dr. Timothy Chuter while a fellow at the University of Rochester.[14]
Society and culture
editSpecial Populations
editOther Animals
editReferences
edit- ^ Nienaber CA, Rousseau H, Eggebrecht H; et al. (December 2009). "Randomized comparison of strategies for type B aortic dissection: the INvestigation of STEnt Grafts in Aortic Dissection (INSTEAD) trial". Circulation. 120 (25): 2519–28. doi:10.1161/CIRCULATIONAHA.109.886408. PMID 19996018.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ a b Greenhalgh RM, Powell JT (2008). "Endovascular repair of abdominal aortic aneurysm". N. Engl. J. Med. 358 (5): 494–501. doi:10.1056/NEJMct0707524. PMID 18234753.
- ^ Eberhardt KM, Sadeghi-Azandaryani M, Worlicek S, Koeppel T, Reiser MF, Treitl M. Treatment of type I endoleaks using transcatheter embolization with onyx. J Endovasc Ther. 2014;21(1):162-71.
- ^ Melas N, Perdikides T, Saratzis A, Saratzis N, Kiskinis D, Deaton DH. Helical EndoStaples enhance endograft fixation in an experimental model using human cadaveric aortas. J Vasc Surg. 2012.
- ^ Avci M, Vos JA, Kolvenbach RR, Verhoeven EL, Perdikides T, Resch TA, et al. The use of endoanchors in repair EVAR cases to improve proximal endograft fixation. J Cardiovasc Surg (Torino). 2012;53(4):419-26.
- ^ Deaton DH. Improving proximal fixation and seal with the HeliFx Aortic EndoAnchor. Semin Vasc Surg. 2012;25(4):187-92.
- ^ Perdikides T, Melas N, Lagios K, Saratzis A, Siafakas A, Bountouris I, et al. Primary endoanchoring in the endovascular repair of abdominal aortic aneurysms with an unfavorable neck. J Endovasc Ther. 2012;19(6):707-15.
- ^ Niepoth WW, de Bruin JL, Yeung KK, Lely RJ, Devrome AN, Wisselink W, et al. A proof-of-concept in vitro study to determine if EndoAnchors can reduce gutter size in chimney graft configurations. J Endovasc Ther. 2013;20(4):498-505.
- ^ De Vries JP, Van De Pavoordt HD, Jordan WD, Jr. Rationale of EndoAnchors in abdominal aortic aneurysms with short or angulated necks. J Cardiovasc Surg (Torino). 2014;55(1):103-7.
- ^ Hogendoorn W, Schlosser FJ, Aruny JE, Indes JE, Sumpio BE, Muhs BE. Successful Treatment of a Proximal Type I Endoleak with HeliFX EndoAnchors. Ann Vasc Surg. 2013.
- ^ "Outpatient endovascular aortic aneurysm repair: experience in 100 consecutive patients". PMID 24045449.
{{cite journal}}: Cite journal requires|journal=(help) - ^ Aho PS (2006). Expectations pf Endovascular Repair of Abdominal Aortic Aneurysm Academic dissertation.
- ^ Volodos' NL, Karpovich IP, Shekhanin VE, et al. A case of distant transfemoral endoprosthesis of the thoracic artery using a self-fixing synthetic prosthesis in traumatic aneurysm]. [Article in Russian] Grudn Khir. 1988 Nov-Dec;(6):84-6. PMID 3220297 [PubMed - indexed for MEDLINE]
- ^ "Transfemoral endovascular aortic graft placement". PMID 8350427.
{{cite journal}}: Cite journal requires|journal=(help)
External links
edit- Society of Interventional Radiology
- Society for Vascular Surgery (U.S.)
- The EVAR Trial: Data and Information
- Information for patients
- Division of Vascular Surgery and Endovascular Therapy at the Baylor College of Medicine
Category:Diseases of the aorta
Category:Interventional radiology
Category:Vascular surgery