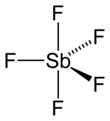
Antimony pentafluoride is the inorganic compound with the formula SbF5. This colourless, viscous liquid is a strong Lewis acid and a component of the superacid fluoroantimonic acid, formed upon mixing liquid HF with liquid SbF5 in 1:1 ratio. It is notable for its strong Lewis acidity and the ability to react with almost all known compounds.[4]
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Antimony pentafluoride
| |||
| Systematic IUPAC name
Pentafluoro-λ5-stibane | |||
| Other names
Antimony(V) fluoride
pentafluoridoantimony | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| ECHA InfoCard | 100.029.110 | ||
| EC Number |
| ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1732 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| SbF5 | |||
| Molar mass | 216.74 g/mol | ||
| Appearance | colorless oily, viscous liquid hygroscopic | ||
| Odor | pungent, sharp | ||
| Density | 2.99 g/cm3 [1] | ||
| Melting point | 8.3 °C (46.9 °F; 281.4 K) | ||
| Boiling point | 149.5 °C (301.1 °F; 422.6 K) | ||
| Reacts | |||
| Solubility | soluble in KF, liquid SO2 | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
Extremely toxic, corrosive, hazardous to health. Releases hydrofluoric acid upon contact with water and biological tissues. Strong oxidizing agent. | ||
| GHS labelling: | |||
     
| |||
| Danger | |||
| H300+H310+H330, H314, H411, H412 | |||
| P260, P261, P264, P270, P271, P273, P280, P301+P312, P301+P330+P331, P303+P361+P353, P304+P312, P304+P340, P305+P351+P338, P310, P312, P321, P330, P363, P391, P405, P501 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | noncombustible | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
270 mg/kg (mouse, subcutaneous) | ||
LC50 (median concentration)
|
270 mg/m3 (mouse, inhalation) | ||
LCLo (lowest published)
|
15 mg/m3 (rat,
inhalation, 2 hours) | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
TWA 0.5 mg/m3 (as Sb)[2] | ||
REL (Recommended)
|
TWA 0.5 mg/m3 (as Sb)[2] | ||
IDLH (Immediate danger)
|
50 mg/m3 | ||
| Safety data sheet (SDS) | ICSC 0220 | ||
| Related compounds | |||
Other anions
|
Antimony pentachloride | ||
Other cations
|
Phosphorus pentafluoride Arsenic pentafluoride Bismuth pentafluoride | ||
Related compounds
|
Antimony trifluoride | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Preparation
editAntimony pentafluoride is prepared by the reaction of antimony pentachloride with anhydrous hydrogen fluoride:[5]
- SbCl5 + 5 HF → SbF5 + 5 HCl
It can also be prepared from antimony trifluoride and fluorine.[6]
Structure and chemical reactions
editIn the gas phase, SbF5 adopts a trigonal bipyramidal structure of D3h point group symmetry (see picture). The material adopts a more complicated structure in the liquid and solid states. The liquid contains polymers wherein each Sb is octahedral, the structure being described with the formula [SbF4(μ-F)2]n ((μ-F) denotes the fact that fluoride centres bridge two Sb centres). The crystalline material is a tetramer, meaning that it has the formula [SbF4(μ-F)]4. The Sb-F bonds are 2.02 Å within the eight-membered Sb4F4 ring; the remaining fluoride ligands radiating from the four Sb centers are shorter at 1.82 Å.[7] The related species PF5 and AsF5 are monomeric in the solid and liquid states, probably due to the smaller sizes of the central atom, which limits their coordination number. BiF5 is a polymer.[8]
SbF5 oxidizes oxygen in the presence of fluorine:[9]
- 2 SbF5 + F2 + 2 O2 → 2 [O2]+[SbF6]−
Antimony pentafluoride by itself, is also a very strong oxidizing agent. Phosphorus burns on contact with it.
SbF5 has also been used in the first discovered chemical reaction that produces fluorine gas from fluoride compounds:
- 4 SbF5 + 2 K2MnF6 → 4 KSbF6 + 2 MnF3 + F2
The driving force for this reaction is the high affinity of SbF5 for F−, which is the same property that recommends the use of SbF5 to generate superacids.
Hexafluoroantimonate
editSbF5 is a strong Lewis acid, exceptionally so toward sources of F− to give the very stable anion [SbF6]−, called hexafluoroantimonate. It is the conjugate base of the superacid fluoroantimonic acid. [SbF6]− is a weakly coordinating anion akin to PF6−. Although it is only weakly basic, [SbF6]− does react with additional SbF5 to give a centrosymmetric adduct:
- SbF5 + [SbF6]− → [Sb2F11]−
The [Sb2F11]− anion is one of the ions found in HF/SbF5 Mixture.
Safety
editSbF5 reacts violently with water. It reacts with many compounds, often releasing dangerous hydrogen fluoride. It is highly toxic and corrosive to the skin and eyes. It is a strong oxidizer.[10][11]
References
edit- ^ Lide, David R., ed. (2006). CRC Handbook of Chemistry and Physics (87th ed.). Boca Raton, FL: CRC Press. ISBN 0-8493-0487-3.
- ^ a b NIOSH Pocket Guide to Chemical Hazards. "#0036". National Institute for Occupational Safety and Health (NIOSH).
- ^ World of Chemicals SDS
- ^ Olah, G. A.; Prakash, G. K. S.; Wang, Q.; Li, X.-y."Antimony(V) Fluoride" in Encyclopedia of Reagents for Organic Synthesis (Ed: L. Paquette) 2004, J. Wiley & Sons, New York. doi:10.1002/047084289X.
- ^ Sabina C. Grund, Kunibert Hanusch, Hans J. Breunig, Hans Uwe Wolf "Antimony and Antimony Compounds" in Ullmann's Encyclopedia of Industrial Chemistry 2006, Wiley-VCH, Weinheim doi:10.1002/14356007.a03_055.pub2
- ^ Handbook of Preparative Inorganic Chemistry, 2nd Ed. Edited by G. Brauer, Academic Press, 1963, NY. Vol. 1. p. 200.
- ^ Edwards, A. J.; Taylor, P. "Crystal structure of Antimony Pentafluoride" Journal of the Chemical Society, Chemical Communications 1971, pp. 1376-7.doi:10.1039/C29710001376
- ^ Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001. ISBN 0-12-352651-5.
- ^ Shamir, J.; Binenboym, J. "Dioxygenyl Salts" Inorganic Syntheses 1973, XIV, 109-122. ISSN 0073-8077
- ^ International Programme on Chemical Safety (2005). "Antimony pentafluoride". Commission of the European Communities (CEC). Retrieved 2010-05-10.
- ^ Barbalace, Kenneth (2006). "Chemical Database - Antimony Pentafluoride". Environmental Chemistry. Retrieved 2010-05-10.