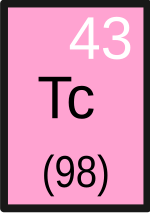
Technetium is a chemical element that has the symbol Tc and the atomic number 43. Pronounced tek-nee-s(h)ee-um, the chemical properties of this silvery grey, radioactive, crystalline transition metal are intermediate between rhenium and manganese. Its short-lived isotope Tc-99m is used in nuclear medicine for a wide variety of diagnostic tests. Tc-99 is used as a gamma ray-free source of beta particles, and its pertechnetate ion (TcO−
4) could find use as an anodic corrosion preventer for steel (this possible use is hindered by technetium's radioactivity). The pertechnetate forms a technetium dioxide layer on the steel surface, this TcO2 formation explains how iron powder can be used to remove technetium from water. It is also possible to absorb technetium on activated carbon.
Dmitri Mendeleev predicted many of the properties of element 43, which he called ekamanganese, well before its actual discovery (see Mendeleev's predicted elements). In 1937 its isotope Tc-97 became the first element to be artificially produced, hence its name (from the Greek τεχνητος, meaning "artificial"). Most technetium produced on Earth is a by-product of fission of uranium-235 in nuclear reactors and is extracted from nuclear fuel rods. No isotope of technetium has a half-life longer than 4.2 million years (Tc-98), so its detection in red giants in 1952 helped bolster the theory that stars can produce heavier elements. On earth, technetium occurs naturally only in uranium ores as a product of spontaneous fission; the quantities are minute but have been measured.