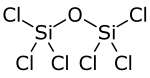
Hexachlorodisiloxane is a chemical compound composed of chlorine, silicon, and oxygen. Structurally, it is the symmetrical ether of two trichlorosilyl groups, and can be synthesized via high-temperature oxidation of silicon tetrachloride:
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.035.504 |
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| Cl6OSi2 | |
| Molar mass | 284.87 g·mol−1 |
| Density | 1.575 g/cm3 |
| Melting point | −33 °C (−27 °F; 240 K) |
| Boiling point | 137 °C (279 °F; 410 K) |
| Hazards | |
| GHS labelling:[1] | |
 
| |
| Danger | |
| H223, H314, H335 | |
| Related compounds | |
Other anions
|
Hexafluorodisiloxane |
Other cations
|
Perchloromethylether |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
At room temperature, it is a colorless liquid that hydrolyzes upon exposure to water to give silicon dioxide and hydrochloric acid: Intense heat evinces a similar decomposition:
Reaction with antimony trifluoride gives the analogous hexafluorodisiloxane.
Sources
edit- Lide, David R., ed. (2009). CRC Handbook of Chemistry and Physics (90th ed.). Boca Raton, Florida: CRC Press. ISBN 978-1-4200-9084-0.
- G. Brauer [Брауэр Г.], ed. (1985). Руководство по неорганическому синтезу [Guide to Inorganic Synthesis] (in Russian). Vol. 3. Moscow: Mir. p. 392.
- K. A. Adrianov [Адрианов К. А.] (1955). Кремнийорганические соединения [Organosilicon Compounds] (in Russian). Moscow: State scientific and technical publishing house of chemical literature. p. 521.
- Booth, Harold Simmons; Osten, Reuben Alexander (July 1945). "The Fluorination of Chlorodisiloxane / Silicon Oxyfluoride". Journal of the American Chemical Society. 67 (7): 1092–1096. doi:10.1021/ja01223a021.
References
edit- ^ "C&L Inventory". echa.europa.eu.
![{\displaystyle {\ce {2SiCl4{}+O2->[ \atop {950-970\,^{\circ }{\text{C}}}]2(SiCl3)2O{}+Cl2}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/492c559a53df8ef9b1a991f6e1e4f7848c017b4e)

![{\displaystyle {\ce {2(SiCl3)2O->[ \atop {\Delta }]SiO2{}+3SiCl4}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/786807d090dd2c58cc244aedd5f271bb6ee94de6)