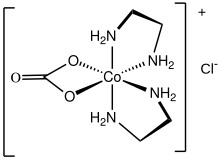
Carbonatobis(ethylenediamine)cobalt(III) chloride is a salt with the formula [CoCO3(en)2]Cl (en = ethylenediamine). It is a red diamagnetic solid that is soluble in water. It is the monochloride salt of a cationic carbonate complex [CoCO3(en)2]+. The chloride ion in this salt readily undergoes ion exchange. The compound is synthesized by the oxidation of a mixture of cobalt(II) chloride, lithium hydroxide, and ethylenediamine in the presence of carbon dioxide:[1]
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C5H16ClCoN4O3 | |
| Molar mass | 274.59 g·mol−1 |
| Appearance | red solid |
| Density | 1.79 g/cm3 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
- CoCl2 + 2 en + CO2 + 0.5 H2O2 + LiOH → [CoCO3(en)2]Cl + H2O + LiCl
The cationic complex is octahedral with C2 symmetry.[2]
The carbonato ligand is readily replaced upon acid hydrolysis. Derivatives include the following complexes: cis- and trans-[CoCl2(en)2]+, cis-[Co(OH)(H2O)3(en)2]2+, cis-[Co(OH2)2(en)2]+, and cis-[Co(NO2)2(en)2]+.[1] Reaction with trifluoromethanesulfonic acid (HOTf) gives [Co(OTf)2(en)2]OTf.[3]
References
edit- ^ a b Springbørg, J.; Schaffer, C. E. (1973). "Dianionobis(ethylenediamine)cobalt(III) Complexes". Inorganic Syntheses. Inorganic Syntheses. Vol. 14. p. 63-77. doi:10.1002/9780470132456.ch14. ISBN 9780470132456.
- ^ García-Granda, S.; Calvo-Pérez, V.; Gómez-Beltrán, F. (1993). "Structure Redetermination of Carbonatobis(ethlenediamine)cobalt(III) Chloride". Acta Crystallographica Section C Crystal Structure Communications. 49 (2): 322–324. doi:10.1107/S0108270192006085.
- ^ Dixon, N. E.; Jackson, W. G.; Lawrance, G. A.; Sargeson, A. M. (1983). "Cobalt(III) Amine Complexes with Coordinated Trifluoromethanesulfonate". Inorganic Syntheses. 22: 103–107. doi:10.1002/9780470132531.ch21.