Cangrelor, sold under the brand name Kengreal among others, is a P2Y12 inhibitor FDA approved as of June 2015 as an antiplatelet drug[5] for intravenous application. Some P2Y12 inhibitors are used clinically as effective inhibitors of adenosine diphosphate-mediated platelet activation and aggregation.[5] Unlike clopidogrel (Plavix), which is a prodrug, cangrelor is an active drug not requiring metabolic conversion.
 | |
| Clinical data | |
|---|---|
| Trade names | Kengreal, Kengrexal |
| Other names | AR-C69931MX |
| AHFS/Drugs.com | Monograph |
| License data | |
| Routes of administration | Intravenous |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 100% (IV) |
| Protein binding | ~97–98%. |
| Metabolism | Rapid deactivation in the circulation (independent of CYP system) |
| Elimination half-life | ~3–6 minutes |
| Excretion | Kidney (58%), Bile duct (35%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
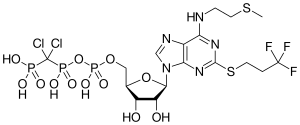
| Formula | C17H25Cl2F3N5O12P3S2 |
| Molar mass | 776.35 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Poor interim results led to the abandonment of the two CHAMPION clinical trials in mid-2009.[6] The BRIDGE study, for short term use prior to surgery, continues.[7] The CHAMPION PHOENIX trial was a randomized study of over 11,000 patients published in 2013. It found usefulness of cangrelor in patients getting cardiac stents. Compared with clopidogrel given around the time of stenting, intravenous ADP-receptor blockade with cangrelor significantly reduced the rate of stent thrombosis and myocardial infarction.[8] Reviewers have questioned the methodology of the trial.[9]
Medical use
editAccording to phase III randomized trials, a cangrelor–clopidogrel combination is safe and has been found to be more effective than standard clopidogrel treatment at reducing ischemic events in the heart, without increasing major bleeding in the treatment of stenotic coronary arteries.[10] The advantages of this drug combination are most prominent in patients with myocardial infarction.[10]
Available antiplatelet drugs have delayed onset and offset of action.[10] Since cangrelor's effects are immediate and quickly reversed, it is a more desirable drug for elective treatment of stenotic coronary arteries, high risk acute coronary syndromes treated with immediate coronary stenting, and for bridging those surgery patients who require P2Y12 inhibition.[10]
Evidence regarding cangrelor therapy is limited by the lack studies assessing cangrelor administration in conjunction with either prasugrel or ticagrelor.[10]
Cangrelor been approved for adults undergoing percutaneous coronary intervention (PCI).[11]
Pharmacology
editCangrelor is a high-affinity, reversible inhibitor of P2Y12 receptors that causes almost complete inhibition of ADP-induced platelet aggregate.[12] It is a modified ATP derivative stable to enzymatic degradation.[12] It does not require metabolic conversion to an active metabolite. This allows cangrelor's immediate effect after infusion,[12] and the therapeutic effects can be maintained with continuous infusion.[13] The pharmacokinetics of cangrelor has allowed it to rapidly achieve steady-state concentrations with a clearance of 50 L/h and a half-life of 2.6 to 3.3 minutes. Cessation of its administration is associated with rapid removal, and normal platelet function is restored within 1 hour.[12][13]
Adverse effects
editDespite fewer bleeding events during cardiac surgery, cangrelor carries the risk of potential autoimmune reactions manifesting as breathlessness.[14] Potential mechanisms for dyspnea following cangrelor treatment include: repeated binding and unbinding cycles, impaired platelet turnover, and lung sequestration or apoptosis of overloaded destructive platelets.[14] The dyspnea risks following cangrelor treatment, suggest a common mechanism linking transfusion-related acute lung injury, dyspnea, and reversible platelet inhibition.[14]
The risk of breathlessness after intravenous cangrelor is smaller when compared with other reversible platelet P2Y12 receptor inhibitors, however, it is still significantly higher when compared to irreversible oral antiplatelet drugs or intravenous glycoprotein IIb/IIIa inhibitors; which do not increase the incidence of breathlessness at all.[14]
Chemistry
editSynthesis
editCangrelor is synthesized starting from 2-thiobarbituric acid and peracetyl-D-ribofuranose.[15][16]
The synthesis starts with the selective S-alkylation of 2-thiobarbituric acid, followed by nitration with nitric acid, leading to the nitrated dihydroxypyrimidine. Treatment with phosphorus oxychloride affords the corresponding dichloropyrimidine. Subsequently, the nitro group is reduced using iron as the reductant, yielding the aniline derivative. This is cyclized to the purine using triethyl orthoformate and hydrochloric acid.
N,O-bis-(trimethylsilyl)acetamide is used to protect the anilinic nitrogen, allowing for the selective N9-alkylation of the compound with peracetyl-D-ribofuranose using trimethylsilyl triflate.[17]
The 5'-OH is converted to a phosphodichloridate using phosphorus oxychloride in triethyl phosphate as the solvent.[18] This is converted to Cangrelor without isolation by reaction with dichloromethylenebis(phosphonic acid) and tributylamine as the base.
References
edit- ^ "Details for: Kengrexal". Health Canada. 30 November 2023. Retrieved 3 March 2024.
- ^ "Notice: Multiple Additions to the Prescription Drug List (PDL) [2023-03-08]". Health Canada. 8 March 2023. Retrieved 21 March 2023.
- ^ "Summary Basis of Decision for Kengrexal". Health Canada. 23 May 2023. Retrieved 5 June 2023.
- ^ "Kengreal- cangrelor injection, powder, lyophilized, for solution". DailyMed. 6 September 2022. Retrieved 3 March 2024.
- ^ a b Norgard NB, Hann CL, Dale GL (2009). "Cangrelor attenuates coated-platelet formation". Clinical and Applied Thrombosis/Hemostasis. 15 (2): 177–182. doi:10.1177/1076029608321437. PMID 18796456. S2CID 23639481.
- ^ CHAMPION Trials With Cangrelor Stopped for Lack of Efficacy
- ^ Napodano J (13 May 2009). "What Cangrelor Failure Means to Medicines". Seeking Alpha.
- ^ Bhatt DL, Stone GW, Mahaffey KW, Gibson CM, Steg PG, Hamm CW, et al. (April 2013). "Effect of platelet inhibition with cangrelor during PCI on ischemic events". The New England Journal of Medicine. 368 (14): 1303–1313. doi:10.1056/NEJMoa1300815. PMID 23473369.
- ^ Lange RA, Hillis LD (April 2013). "The duel between dual antiplatelet therapies". The New England Journal of Medicine. 368 (14): 1356–1357. doi:10.1056/NEJMe1302504. PMID 23473370.
- ^ a b c d e Kubica J, Kozinski M, Navarese EP, Tantry U, Kubica A, Siller-Matula JM, et al. (May 2014). "Cangrelor: an emerging therapeutic option for patients with coronary artery disease". Current Medical Research and Opinion. 30 (5): 813–828. doi:10.1185/03007995.2014.880050. PMID 24393016. S2CID 30451326.
- ^ "FDA approves new antiplatelet drug used during heart procedure" (Press release). U.S. Food and Drug Administration. 22 June 2015. Archived from the original on 23 June 2015.
- ^ a b c d Angiolillo DJ, Capranzano P (August 2008). "Pharmacology of emerging novel platelet inhibitors". American Heart Journal. 156 (2 Suppl): S10–S15. doi:10.1016/j.ahj.2008.06.004. PMID 18657681.
- ^ a b Bhatt DL, Stone GW, Mahaffey KW, Gibson CM, Steg PG, Hamm CW, et al. (April 2013). "Effect of platelet inhibition with cangrelor during PCI on ischemic events". The New England Journal of Medicine. 368 (14): 1303–1313. doi:10.1056/nejmoa1300815. PMID 23473369.
- ^ a b c d Serebruany VL, Sibbing D, DiNicolantonio JJ (2014). "Dyspnea and reversibility of antiplatelet agents: ticagrelor, elinogrel, cangrelor, and beyond". Cardiology. 127 (1): 20–24. doi:10.1159/000354876. PMID 24192670. S2CID 207707382.
- ^ Flick AC, Ding HX, Leverett CA, Kyne RE, Liu KK, Fink SJ, O'Donnell CJ (August 2017). "Synthetic Approaches to the New Drugs Approved During 2015". Journal of Medicinal Chemistry. 60 (15): 6480–6515. doi:10.1021/acs.jmedchem.7b00010. PMID 28421763.
- ^ CN105481922A, 周峰; 金华 & 郑永勇 et al., "一种坎格雷洛中间体的制备方法", issued 2016-04-13
- ^ Almond MR, Collins JL, Reitter BE, Rideout JL, Freeman GA, St Clair MH (7 October 1991). "Synthesis of 2-amino-9-(3′-azido-2′,3′-dideoxy-beta-D-erythro-pentofuranosyl)-6-methoxy-9H-purine (AzddMAP) and AzddGuo". Tetrahedron Letters. 32 (41): 5745–5748. doi:10.1016/S0040-4039(00)93545-7. ISSN 0040-4039.
- ^ Yoshikawa M, Kato T, Takenishi T (December 1967). "A novel method for phosphorylation of nucleosides to 5'-nucleotides". Tetrahedron Letters. 8 (50): 5065–5068. doi:10.1016/S0040-4039(01)89915-9. PMID 6081184.