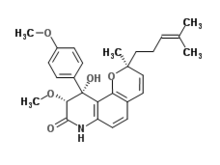
Yaequinolone J1 is an antibiotic made by Penicillium.[1]
| |
| Names | |
|---|---|
| IUPAC name
(2S,9R,10R)-10-Hydroxy-9-methoxy-10-(4-methoxyphenyl)-2-methyl-2-(4-methyl-3-penten-1-yl)-2,7,9,10-tetrahydro-8H-pyrano[2,3-f]quinolin-8-one
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C27H31NO5 | |
| Molar mass | 449.547 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Total syntheses of yaequinolone J1 edit
An asymmetric total synthesis of yaequinolone J1 has been published in 2018 by V. Vece, S. Jakkepally and S. Hanessian.[2] In 2020, a five-step synthesis of yaequinolone J1 was reported.[3]
References edit
- ^ Uchida, R; Imasato, R; Shiomi, K; Tomoda, H; Omura, S (2005). "Yaequinolones J1 and J2, novel insecticidal antibiotics from Penicillium sp. FKI-2140". Org Lett. 7 (25): 5701–4. doi:10.1021/ol052458h. PMID 16321026.
- ^ Vece, V; Jakkepally, S; Hanessian, S (2018). "Total Synthesis and Absolute Stereochemical Assignment of the Insecticidal Metabolites Yaequinolones J1 and J2". Org. Lett. 20 (14): 4277–4280. doi:10.1021/acs.orglett.8b01701. PMID 29975546. S2CID 49710214.
- ^ Schwan, J; Kleoff, M; Heretsch, P; Christmann, M (2020). "Five-Step Synthesis of Yaequinolones J1 and J2". Org. Lett. 22 (2): 475–479. doi:10.1021/acs.orglett.9b04455. PMID 31909626.