 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Claritin, Claratyne, Clarityn, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a697038 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| Drug class | Second-generation antihistamine |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | almost 100% |
| Protein binding | 97–99% |
| Metabolism | liver (CYP2D6- and 3A4-mediated) |
| Elimination half-life | 8 hours, active metabolite desloratadine 27 hours |
| Excretion | 40% as conjugated metabolites into urine Similar amount into the feces |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
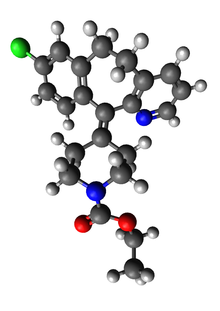
| Formula | C22H23ClN2O2 |
| Molar mass | 382.89 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
- ^ "Loratadine Use During Pregnancy". Drugs.com. 10 February 2020. Retrieved 10 April 2020.
- ^ "Claritin Allergy Product information". Health Canada. 25 April 2012. Retrieved 7 June 2022.
- ^ "Clarityn Allergy 10mg Tablets (P & GSL) - Patient Information Leaflet (PIL)". (emc). 30 August 2019. Archived from the original on 10 April 2020. Retrieved 10 April 2020.