 | |
| Clinical data | |
|---|---|
| Trade names | Caprelsa |
| Other names | ZD6474 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a611037 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth[1] |
| Drug class | Kinase inhibitor[1] |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | 90–96% |
| Metabolism | CYP3A4, FMO1, FMO3 |
| Elimination half-life | 19 days (mean)[2] |
| Excretion | 44% faeces, 25% urine |
| Identifiers | |
| |
| Chemical and physical data | |
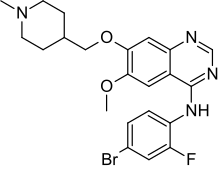
| Formula | C22H24BrFN4O2 |
| Molar mass | 475.362 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Vandetanib, sold under the brand name Caprelsa, is an anti-cancer medication used to treat a type of thyroid cancer, specifically medullary thyroid cancer.[1] It increases the amount of time before the disease worsens.[1] It is taken by mouth.[1]
Common side effects include acne, high blood pressure, headache, low calcium, low blood sugar, and diarrhea.[1] Other side effects include QT prolongation, sun sensitivity, hair loss, swelling, and corneal deposits.[1][3] Use during pregnancy or breastfeeding may harm the baby.[4] It works as a kinase inhibitor, mainly of the vascular endothelial growth factor receptor 2 (VEGFR2) and the epidermal growth factor receptor (EGFR).[5]
Vandetanib was approved for medical use in the United States in 2011.[1] In the United Kingdom it costs the NHS £5,000 per month as of 2020.[3] In the United States this amount costs about 16,000 USD as of 2021;[6] while in Canada it cost about 5,900 CAD per month as of 2017.[7]
References
edit- ^ a b c d e f g h i j k l "Vandetanib Monograph for Professionals". Drugs.com. Archived from the original on 24 July 2012. Retrieved 6 August 2021.
- ^ a b "Caprelsa- vandetanib tablet, film coated". DailyMed. 19 June 2020. Archived from the original on 27 October 2020. Retrieved 8 December 2020.
- ^ a b c BNF (80 ed.). BMJ Group and the Pharmaceutical Press. September 2020 – March 2021. p. 1061. ISBN 978-0-85711-369-6.
{{cite book}}: CS1 maint: date format (link) - ^ "Vandetanib (Caprelsa) Use During Pregnancy". Drugs.com. Archived from the original on 29 October 2020. Retrieved 6 August 2021.
- ^ "Definition of vandetanib". NCI Drug Dictionary. National Cancer Institute. 2011-02-02. Archived from the original on 2018-01-24. Retrieved 2021-05-02.
- ^ "Caprelsa Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 27 August 2021. Retrieved 6 August 2021.
- ^ "pCODR Expert Review Committee Final Recommendation" (PDF). CADTH. Archived (PDF) from the original on 19 October 2018. Retrieved 6 August 2021.