 | |
| Clinical data | |
|---|---|
| Trade names | Daybue |
| Other names | NNZ-2566 |
| License data |
|
| Routes of administration | By mouth |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 84% |
| Metabolism | Insignificant |
| Elimination half-life | ~ 1.5 h |
| Excretion | Urine |
| Identifiers | |
| |
| Chemical and physical data | |
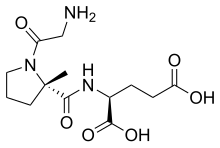
| Formula | C13H21N3O6 |
| Molar mass | 315.326 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Trofinetide, sold under the brand name Daybue, is a medication used to treat Rett syndrome.[1] It is used in people at least two years old.[1] It is taken by mouth.[1]
Common side effects include diarrhea and vomiting.[1] Weight loss may occur.[1] use is not recommended in those with significant kidney problems.[1] How it works is unclear.[1]
Trofinetide was approved for medical use in the United States in 2023.[1] For those who weight less than 12 kg it will cost about 385,000 USD per year, while in those who more than 50 kg it will cost about 924,000 USD per year in the United States as of 2023.[2]
References edit
- ^ a b c d e f g h i j k l "DailyMed - DAYBUE- trofinetide solution". dailymed.nlm.nih.gov. Archived from the original on 2 July 2023. Retrieved 12 June 2023.
- ^ "FDA Approves First Treatment for Rett syndrome". Formulary Watch. 13 March 2023. Archived from the original on 22 May 2023. Retrieved 12 June 2023.