 | |
| Clinical data | |
|---|---|
| Trade names | Evrysdi |
| Other names | RG7916; RO7034067 |
| AHFS/Drugs.com | Monograph |
| License data | |
| Routes of administration | By mouth |
| Drug class | SMN2-directed RNA splicing modifier[1] |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| Chemical and physical data | |
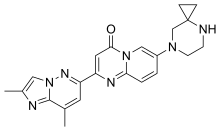
| Formula | C22H23N7O |
| Molar mass | 401.474 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Risdiplam, sold under the brand name Evrysdi, is a medication used to treat spinal muscular atrophy (SMA).[1] This includes type 1, type 2, and type 3 disease.[3] It is used in those who are at least two months old.[1] It is taken by mouth.[1]
Common side effects included fever, diarrhea, rash, pneumonia, and vomiting.[1] Use in pregnancy may harm the baby.[4] It is a survival of motor neuron 2-directed RNA splicing modifier.[1]
Risdiplam was approved for medical use in the United States in 2020 and Europe in 2021.[5][3] In the United States 60 mg costs about 11,700 USD as of 2021.[6] In the United Kingdom this amount costs the NHS about £7900.[7]
References
edit- ^ a b c d e f g h i j Food and Drugs Administration (FDA) (18 August 2020). "Evrysdi- risdiplam powder, for solution". DailyMed. Bethesda, Maryland, United States: MedLine by the National Library of Medicine (NLM) of the United States National Institutes of Health (NIH). Archived from the original on 29 August 2021. Retrieved 24 September 2020.
- ^ O'Keefe, Lindsey (7 August 2020). "FDA Approves Oral Treatment for Spinal Muscular Atrophy" (Press release). Silver Spring, Maryland, United States of America: United States Food and Drug Administration (FDA). FDA Newsroom Department. Archived from the original on 11 August 2020. Retrieved 7 August 2020. This article incorporates text from this source, which is in the public domain.
- ^ a b "Evrysdi". Archived from the original on 6 October 2021. Retrieved 18 October 2021.
- ^ "Risdiplam (Evrysdi) Use During Pregnancy". Drugs.com. Archived from the original on 30 October 2020. Retrieved 18 October 2021.
- ^ "Risdiplam Monograph for Professionals". Drugs.com. Archived from the original on 20 October 2021. Retrieved 18 October 2021.
- ^ "Evrysdi Prices, Coupons & Patient Assistance Programs". Drugs.com. Retrieved 18 October 2021.
- ^ "Risdiplam". SPS - Specialist Pharmacy Service. 6 February 2019. Archived from the original on 18 October 2021. Retrieved 18 October 2021.