 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /priˈɡæbəlɪn/ |
| Trade names | Lyrica, others[1] |
| Other names | 3-isobutyl GABA, (S)-3-isobutyl-γ-aminobutyric acid |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a605045 |
| License data |
|
| Pregnancy category |
|
| Dependence liability | Physical: Moderate[3] Psychological: Moderate[3] |
| Addiction liability | Low[3] |
| Routes of administration | By mouth |
| Drug class | Gabapentinoid |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | High (≥90% rapidly absorbed; administration with food has no significant effect on bioavailability)[4] |
| Protein binding | <1%[5] |
| Metabolites | N-methylpregabalin[4] |
| Onset of action | May occur within a week (pain)[6] |
| Elimination half-life | 6.3–11.5 hours[7][8][9] |
| Duration of action | Unknown[10] |
| Excretion | Kidney |
| Identifiers | |
| |
| Chemical and physical data | |
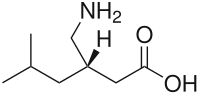
| Formula | C8H17NO2 |
| Molar mass | 159.229 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Pregabalin, marketed under the brand name Lyrica among others, is a medication used to treat epilepsy, neuropathic pain, fibromyalgia, restless leg syndrome, and generalized anxiety disorder.[11][12][13] Its use in epilepsy is as an add-on therapy for partial seizures.[11] When used before surgery, it reduces pain but results in greater sedation and visual disturbances.[16] It is taken by mouth.[11]
Common side effects include headache, dizziness, sleepiness, confusion, trouble with memory, poor coordination, dry mouth, problem with vision, and weight gain.[11][14] Serious side effects may include angioedema, drug misuse, and an increased suicide risk.[11] When pregabalin is taken at high doses over a long period of time, addiction may occur, but if taken at usual doses the risk is low.[3] Use during the first trimester of pregnancy may slightly raise the risk of birth defects.[17] Use during breastfeeding is of unclear safety.[18] Pregabalin is a gabapentinoid and acts by inhibiting certain calcium channels.[19][20]
Pregabalin was approved for medical use in the United States in 2004.[11] It was developed as a successor to gabapentin.[21] It is available as a generic medication in a number of countries, including the United States as of 2019.[14][22][23] In the US the wholesale cost is about US$11 per month as of 2019.[24] While in the United Kingdom a similar dose costs the NHS about £6 as of 2018.[14] In 2017, it was the 72nd most prescribed medication in the United States with more than 11 million prescriptions.[25][26] In the US, Pregabalin is a Schedule V controlled substance.[11] It is a Class C controlled substance in the UK.[27] In France it is the most commonly forged prescription.[28]
References edit
- ^ "Pregabalin - Drugs.com". www.drugs.com. Archived from the original on August 28, 2019. Retrieved November 7, 2016.
- ^ a b "Pregabalin Use During Pregnancy". Drugs.com. June 12, 2018. Archived from the original on March 21, 2019. Retrieved January 21, 2020.
- ^ a b c d Schifano, Fabrizio (2014). "Misuse and Abuse of Pregabalin and Gabapentin: Cause for Concern?". CNS Drugs. 28 (6): 491–6. doi:10.1007/s40263-014-0164-4. PMID 24760436.
- ^ a b "Summary of product characteristics" (PDF). European Medicines Agency. March 6, 2013. Archived (PDF) from the original on September 16, 2018. Retrieved May 6, 2013.
- ^ "A comparison of the pharmacokinetics and pharmacodynamics of pregabalin and gabapentin". Clinical Pharmacokinetics. 49 (10): 661–669. 2010. doi:10.2165/11536200-000000000-00000. PMID 20818832.
{{cite journal}}: Cite uses deprecated parameter|authors=(help) - ^ "Pregabalin (Professional Patient Advice) - Drugs.com". www.drugs.com. Archived from the original on July 31, 2020. Retrieved November 7, 2016.
- ^ "Pregabalin (Professional Patient Advice)". Drugs.com. Archived from the original on July 31, 2020. Retrieved August 4, 2020.
- ^ Hantson, P; Courtois, F; Borrey, D; Haufroid, V (2014). "Pregabalin-associated myoclonic encephalopathy without evidence of drug accumulation in a patient with acute renal failure". Indian Journal of Nephrology. 24 (1): 48–50. doi:10.4103/0971-4065.125102. PMC 3927193. PMID 24574633.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Randinitis, Edward J.; Posvar, Edward L.; Alvey, Christine W.; Sedman, Allen J.; Cook, Jack A.; Bockbrader, Howard N. (2003). "Pharmacokinetics of Pregabalin in Subjects with Various Degrees of Renal Function". The Journal of Clinical Pharmacology. 43 (3): 277–83. doi:10.1177/0091270003251119. PMID 12638396.
- ^ Lilley, Linda Lane; Collins, Shelly Rainforth; Snyder, Julie S. (2015). Pharmacology and the Nursing Process. Elsevier Health Sciences. p. 227. ISBN 9780323358286.
- ^ a b c d e f g h i "Pregabalin". The American Society of Health-System Pharmacists. Archived from the original on December 2, 2019. Retrieved February 3, 2019.
- ^ a b Frampton, James E. (2014). "Pregabalin: A Review of its Use in Adults with Generalized Anxiety Disorder". CNS Drugs. 28 (9): 835–54. doi:10.1007/s40263-014-0192-0. PMID 25149863.
- ^ a b Iftikhar, IH; Alghothani, L; Trotti, LM (December 2017). "Gabapentin enacarbil, pregabalin and rotigotine are equally effective in restless legs syndrome: a comparative meta-analysis". European Journal of Neurology. 24 (12): 1446–1456. doi:10.1111/ene.13449. PMID 28888061.
- ^ a b c d British National Formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. p. 323. ISBN 9780857113382.
- ^ "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on November 17, 2020. Retrieved September 9, 2020.
- ^ Mishriky, B. M.; Waldron, N. H.; Habib, A. S. (January 2015). "Impact of pregabalin on acute and persistent postoperative pain: a systematic review and meta-analysis". British Journal of Anaesthesia. 114 (1): 10–31. doi:10.1093/bja/aeu293. ISSN 1471-6771. PMID 25209095.
- ^ "4. Nervous system". BNF (84 ed.). London: BMJ Group and the Pharmaceutical Press. September 2022 – March 2023. pp. 353–355. ISBN 978-0-85711-432-7.
- ^ "Pregabalin Use During Pregnancy". Drugs.com. Archived from the original on March 21, 2019. Retrieved February 4, 2019.
- ^ "Alpha2delta ligands, gabapentin, pregabalin and mirogabalin: a review of their clinical pharmacology and therapeutic use". Expert Review of Neurotherapeutics. 16 (11): 1263–1277. 2016. doi:10.1080/14737175.2016.1202764. PMID 27345098.
{{cite journal}}: Cite uses deprecated parameter|authors=(help) - ^ "Acute modulation of calcium currents and synaptic transmission by gabapentinoids". Channels (Austin). 4 (6): 490–496. 2010. doi:10.4161/chan.4.6.12864. PMID 21150315.
{{cite journal}}: Cite uses deprecated parameter|authors=(help) - ^ Kaye, Alan David; Vadivelu, Nalini; Urman, Richard D. (2014). Substance Abuse: Inpatient and Outpatient Management for Every Clinician. Springer. p. 324. ISBN 9781493919512. Archived from the original on March 27, 2019. Retrieved August 4, 2020.
- ^ "Generic Lyrica Availability". Drugs.com. Archived from the original on March 27, 2019. Retrieved February 4, 2019.
- ^ "FDA approves first generics of Lyrica". U.S. Food and Drug Administration (FDA) (Press release). September 11, 2019. Archived from the original on June 9, 2020. Retrieved October 27, 2019.
- ^ "NADAC as of 2019-10-23". Centers for Medicare and Medicaid Services. Archived from the original on October 27, 2019. Retrieved October 27, 2019.
- ^ "The Top 300 of 2020". ClinCalc. Archived from the original on March 18, 2020. Retrieved April 2, 2020.
- ^ "Pregabalin". ClinCalc. December 23, 2019. Archived from the original on July 8, 2020. Retrieved April 2, 2020.
- ^ "Pregabalin and gabapentin to be controlled as Class C drugs". GOV.UK. October 15, 2018. Archived from the original on November 12, 2020. Retrieved April 2, 2020.
- ^ Moisset, X; Bouhassira, D; Attal, N (September 2021). "French guidelines for neuropathic pain: An update and commentary". Revue neurologique. 177 (7): 834–837. doi:10.1016/j.neurol.2021.07.004. PMID 34332778.