 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Numorphan, Numorphone, Opana, others |
| Other names | 14-Hydroxydihydromorphinone |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a610022 |
| License data |
|
| Routes of administration | By mouth, intravenous, subcutaneous, intramuscular |
| Drug class | Opioid[1] |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | by mouth: 10% Buccal: 28% Sublingual: 37.5% Intranasal: 43%[3] IV, IM & IT: 100%[4] |
| Protein binding | 10%[4] |
| Metabolism | Liver (CYP3A4, glucuronidation)[4] |
| Onset of action | By injection: Within 15 min |
| Elimination half-life | 7–9 hours[4] Duration of Action: 6-8 hours Orally, 4-6 hrs Parenteral. |
| Duration of action | Immediate release: 5 hrs Extended release: 12 hrs |
| Excretion | Urine, feces[4] |
| Identifiers | |
| |
| Chemical and physical data | |
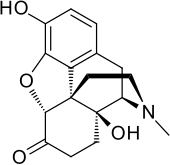
| Formula | C17H19NO4 |
| Molar mass | 301.342 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Oxymorphone, sold under the brand names Opana among others, is an opioid used to treat moderate to severe pain.[1] It is available by mouth and by injection.[1] Effects begin within 15 minutes following injection.[1] It lasts about 5 hours and 12 hours for extended-release tablets.[5]
Common side effects include nausea, constipation, sleepiness, itchiness, and confusion.[1] Other side effects may include respiratory depression, adrenal insufficiency, and low blood pressure.[1] Use during pregnancy may result in dependence in the baby, with use during delivery associated with breathing problems.[1] It has a high risk of abuse.[1]
Oxymorphone was made in Germany in 1914.[6] It was patented in 1955 and approved for medical use in 1959.[7] It is available as a generic medication.[1] In the United States the 5 mg tablets costs about 1.35 USD as of 2021.[8] On the illicit market this amount cost about 7.50 USD as of 2010.[9]
References edit
- ^ a b c d e f g h i j k "OxyMORphone Monograph for Professionals". Drugs.com. Archived from the original on 4 March 2021. Retrieved 10 November 2021.
- ^ "Drugs@FDA: FDA Approved Drug Products". www.accessdata.fda.gov. Archived from the original on 2 November 2017. Retrieved 7 November 2017.
- ^ Hussain MA, Aungst BJ (August 1997). "Intranasal absorption of oxymorphone". Journal of Pharmaceutical Sciences. 86 (8): 975–6. doi:10.1021/js960513x. PMID 9269879.
- ^ a b c d e Davis MP, Glare PA, Hardy J (2009) [2005]. Opioids in Cancer Pain (2nd ed.). Oxford, UK: Oxford University Press. pp. Chapter 17. ISBN 978-0-19-157532-7. Archived from the original on 2021-10-25. Retrieved 2021-10-21.
- ^ Sloan P (August 2008). "Review of oral oxymorphone in the management of pain". Therapeutics and Clinical Risk Management. 4 (4): 777–87. doi:10.2147/tcrm.s1784. PMC 2621383. PMID 19209260.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Sinatra, Raymond S.; Jahr, Jonathan S.; Watkins-Pitchford, J. Michael (14 October 2010). The Essence of Analgesia and Analgesics. Cambridge University Press. p. 123. ISBN 978-1-139-49198-3. Archived from the original on 10 November 2021. Retrieved 10 November 2021.
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 52X. ISBN 9783527607495. Archived from the original on 2021-10-25. Retrieved 2021-10-21.
- ^ "Oxymorphone Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 19 March 2016. Retrieved 10 November 2021.
- ^ Surratt, H.; Kurtz, S.; Cicero, T.; Dart, R.; Baker, G.; Vorsanger, G. (April 2013). "Street prices of prescription opioids diverted to the illicit market: data from a national surveillance program". The Journal of Pain. 14 (4): S40. doi:10.1016/j.jpain.2013.01.455.