 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Nebilet, Bystolic, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a608029 |
| License data | |
| Routes of administration | By mouth |
| Drug class | Beta blocker[1] |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | 98% |
| Metabolism | Liver (CYP2D6-mediated) |
| Elimination half-life | 12 hours[2] |
| Excretion | Kidney and fecal |
| Identifiers | |
| |
| Chemical and physical data | |
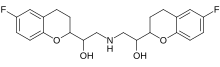
| Formula | C22H25F2NO4 |
| Molar mass | 405.442 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Nebivolol is a beta blocker used to treat high blood pressure and heart failure.[1] As with other β-blockers, it is generally a less preferred treatment for high blood pressure.[3] It may be used by itself or with other blood pressure medication.[3] It is taken by mouth.[3]
Common side effects include dizziness, feeling tired, nausea, and headaches.[3] Serious side effects may include heart failure and bronchospasm.[3] Its use in pregnancy and breastfeeding is not recommended.[1][5] It works by blocking β1-adrenergic receptors in the heart and dilating blood vessels.[3][6]
Nebivolol was patented in 1983 and came into medical use in 1997.[7] It is available as a generic medication in the United Kingdom.[1] A month supply in the United Kingdom costs the NHS about £3 as of 2020.[1] In the United States the wholesale cost of this amount is about US$174.00.[8] In 2017, it was the 144th most commonly prescribed medication in the United States, with more than four million prescriptions.[9][10]
References
edit- ^ a b c d e f BNF (80 ed.). BMJ Group and the Pharmaceutical Press. September 2020 – March 2021. p. 167. ISBN 978-0-85711-369-6.
- ^ Benowitz, Neal L. (2020). "11. Antihypertensive agents". In Katzung, Bertram G.; Trevor, Anthony J. (eds.). Basic and Clinical Pharmacology (15th ed.). New York: McGraw-Hill. p. 183. ISBN 978-1-260-45231-0. Archived from the original on 10 October 2021. Retrieved 5 December 2021.
- ^ a b c d e f g "Nebivolol Hydrochloride Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Archived from the original on 19 October 2012. Retrieved 3 March 2019.
- ^ "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 26 February 2021. Retrieved 5 September 2020.
- ^ "Nebivolol Pregnancy and Breastfeeding Warnings". Drugs.com. Archived from the original on 30 October 2020. Retrieved 3 March 2019.
- ^ de Boer RA, Voors AA, van Veldhuisen DJ (July 2007). "Nebivolol: third-generation beta-blockade". Expert Opin Pharmacother. 8 (10): 1539–50. doi:10.1517/14656566.8.10.1539. PMID 17661735.
- ^ Fischer, Jnos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 462. ISBN 9783527607495. Archived from the original on 1 March 2019. Retrieved 1 March 2019.
- ^ "Bystolic Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 15 February 2021. Retrieved 15 February 2021.
- ^ "The Top 300 of 2020". ClinCalc. Archived from the original on 12 February 2021. Retrieved 11 April 2020.
- ^ "Nebivolol Hydrochloride - Drug Usage Statistics (2008-2018)". clincalc.com. Archived from the original on 17 February 2021. Retrieved 17 February 2021.
{{cite web}}:|archive-date=/|archive-url=timestamp mismatch; 13 January 2021 suggested (help)