 | |
| Clinical data | |
|---|---|
| Trade names | Firazyr |
| Other names | Hoe 140, JE 049[1] |
| AHFS/Drugs.com | Monograph |
| License data | |
| Pregnancy category |
|
| Routes of administration | Subcutaneous |
| Drug class | Bradykinin B2 receptors inhibitor[2] |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| Chemical and physical data | |
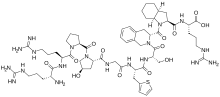
| Formula | C59H89N19O13S |
| Molar mass | 1304.54 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Icatibant, sold under the brand name Firazyr, is a medication used to treat attacks of hereditary angioedema (HAE) in people with insufficient C1-esterase inhibitor.[2][3] It does not appear effective for angioedema due to ACE inhibitors.[4][5] It is given by injection under the skin.[3]
Common side effects include redness, itching, and pain at the site of injection.[3] Other side effects may include fever, headache, and nausea.[6] Safety in pregnancy is unclear.[7] It is a bradykinin B2 receptors inhibitor.[2]
Icatibant was approved for medical use in Europe in 2008 and the United States in 2011.[3][8] In the United Kingdom it costs the NHS about £1,400 per dose.[6] In the United States this amount costs about 3,600 USD.[9]
References edit
- ^ "Icatibant: HOE 140, JE 049, JE049". Drugs in R&D. 5 (6): 343–8. 2004. doi:10.2165/00126839-200405060-00006. PMID 15563238.
- ^ a b c d e f "Firazyr- icatibant acetate injection, solution". DailyMed. 16 December 2019. Archived from the original on 3 August 2020. Retrieved 17 April 2020.
- ^ a b c d e f "Firazyr EPAR". European Medicines Agency (EMA). Archived from the original on 12 April 2020. Retrieved 17 April 2020.
- ^ Jeon, J; Lee, YJ; Lee, SY (October 2019). "Effect of icatibant on angiotensin-converting enzyme inhibitor-induced angioedema: A meta-analysis of randomized controlled trials". Journal of clinical pharmacy and therapeutics. 44 (5): 685–692. doi:10.1111/jcpt.12997. PMID 31290163.
- ^ Sinert R, Levy P, Bernstein JA, Body R, Sivilotti ML, Moellman J, et al. (September–October 2017). "Randomized Trial of Icatibant for Angiotensin-Converting Enzyme Inhibitor-Induced Upper Airway Angioedema". The Journal of Allergy and Clinical Immunology. In Practice. 5 (5): 1402–1409.e3. doi:10.1016/j.jaip.2017.03.003. PMID 28552382.
- ^ a b BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 306. ISBN 978-0857114105.
- ^ "Icatibant (Firazyr) Use During Pregnancy". Drugs.com. Archived from the original on 4 December 2020. Retrieved 25 November 2021.
- ^ "Icatibant Monograph for Professionals". Drugs.com. Archived from the original on 23 January 2021. Retrieved 25 November 2021.
- ^ "Icatibant Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 24 January 2021. Retrieved 25 November 2021.