 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Epidiolex, Epidyolex |
| Other names | CBD, cannabidiolum, (−)-cannabidiol[1] |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a618051 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth[3] |
| Drug class | Cannabinoid[4] |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | • By mouth: 6% (fasted)[9] • Inhaled: 31% (11–45%)[10] |
| Elimination half-life | 18–32 hours[11] |
| Identifiers | |
| |
| Chemical and physical data | |
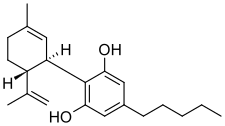
| Formula | C21H30O2 |
| Molar mass | 314.469 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 66 °C (151 °F) |
| Solubility in water | insoluble |
| |
| |
| (verify) | |
Cannabidiol (CBD) is a cannabinoid and medication used to treat seizures in tuberous sclerosis complex, Lennox-Gastaut and Dravet syndrome.[12][13][4] It is used together with clobazam.[12] It is taken by mouth.[12] Evidence is insufficient to support its use for anxiety, psychosis, or pain as of 2019.[14][15]
Common side effects include agitation, diarrhea, tiredness, trouble sleeping, and rash.[12] Other side effects may include liver problems and suicide.[3] Use in pregnancy may harm the baby.[3] Its mechanism of action is not entirely clear.[8] It does not have the same psychoactive properties as tetrahydrocannabinol (THC).[16]
Cannabidiol was discovered in 1940.[4] It was approved for medical use in the United States in 2018 and Europe in 2019.[3][8] In the United States 300 ml of 100 mg/ml solution costs about 4,600 USD as of 2021.[17] It made from the Cannabis sativa plant.[16] Some people use it in the form of CBD oil, which general contains low levels of THC.[15]
References edit
- ^ "cannabidiol (CHEBI:69478)". www.ebi.ac.uk. Archived from the original on 2021-05-12. Retrieved 2021-10-30.
- ^ "Epidyolex". Therapeutic Goods Administration (TGA). 29 September 2020. Archived from the original on 30 October 2021. Retrieved 30 September 2020.
- ^ a b c d e "Epidiolex- cannabidiol solution". DailyMed. 26 August 2020. Archived from the original on 25 February 2021. Retrieved 11 September 2020.
- ^ a b c Strongin, Robert M.; Meehan-Atrash, Jiries; Vialpando, Monica (21 November 2021). Recent Advances in the Science of Cannabis. CRC Press. p. 80. ISBN 978-1-000-46771-0. Archived from the original on 11 January 2022. Retrieved 7 January 2022.
- ^ "Epidyolex 100 mg/ml oral solution - Summary of Product Characteristics (SmPC)". (emc). 28 August 2020. Archived from the original on 16 February 2021. Retrieved 11 September 2020.
- ^ "Sativex Oromucosal Spray - Summary of Product Characteristics (SmPC)". (emc). 25 August 2020. Archived from the original on 4 February 2021. Retrieved 11 September 2020.
- ^ "LeafPro CBDmed Oil FS QP 20%- cannabidiol oil". DailyMed. 9 March 2020. Archived from the original on 1 November 2021. Retrieved 11 September 2020.
- ^ a b c "Epidyolex EPAR". European Medicines Agency (EMA). 24 June 2019. Archived from the original on 9 August 2021. Retrieved 11 September 2020. Text was copied from this source which is copyrighted by the European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ^ Perucca E, Bialer M (5 June 2020). "Critical Aspects Affecting Cannabidiol Oral Bioavailability and Metabolic Elimination, and Related Clinical Implications". CNS Drugs. 34 (8): 795–800. doi:10.1007/s40263-020-00741-5. PMID 32504461. S2CID 219313952.
- ^ Scuderi C, Filippis DD, Iuvone T, Blasio A, Steardo A, Esposito G (May 2009). "Cannabidiol in medicine: a review of its therapeutic potential in CNS disorders". Phytotherapy Research (Review). 23 (5): 597–602. doi:10.1002/ptr.2625. PMID 18844286. S2CID 21836765. Archived from the original on 2021-04-11. Retrieved 2021-10-30.
- ^ Devinsky O, Cilio MR, Cross H, Fernandez-Ruiz J, French J, Hill C, et al. (June 2014). "Cannabidiol: pharmacology and potential therapeutic role in epilepsy and other neuropsychiatric disorders". Epilepsia. 55 (6): 791–802. doi:10.1111/epi.12631. PMC 4707667. PMID 24854329.
- ^ a b c d e f BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 326. ISBN 978-0857114105.
- ^ "Cannabidiol Monograph for Professionals". Drugs.com. Archived from the original on 27 October 2020. Retrieved 7 January 2022.
- ^ Black N, Stockings E, Campbell G, Tran LT, Zagic D, Hall WD, et al. (December 2019). "Cannabinoids for the treatment of mental disorders and symptoms of mental disorders: a systematic review and meta-analysis". The Lancet. Psychiatry. 6 (12): 995–1010. doi:10.1016/S2215-0366(19)30401-8. PMC 6949116. PMID 31672337.
- ^ a b VanDolah HJ, Bauer BA, Mauck KF (September 2019). "Clinicians' Guide to Cannabidiol and Hemp Oils". Mayo Clinic Proceedings. 94 (9): 1840–1851. doi:10.1016/j.mayocp.2019.01.003. PMID 31447137.
- ^ a b Campos AC, Moreira FA, Gomes FV, Del Bel EA, Guimarães FS (December 2012). "Multiple mechanisms involved in the large-spectrum therapeutic potential of cannabidiol in psychiatric disorders". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences (Review). 367 (1607): 3364–78. doi:10.1098/rstb.2011.0389. PMC 3481531. PMID 23108553.
- ^ "Epidiolex Prices and Epidiolex Coupons - GoodRx". GoodRx. Archived from the original on 5 August 2021. Retrieved 7 January 2022.