 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Solian, Barhemsys, Socian, Deniban, others |
| Other names | APD421 |
| AHFS/Drugs.com | Monograph |
| License data |
|
| Pregnancy category | |
| Routes of administration | By mouth, intravenous |
| Drug class | Atypical antipsychotics[3] |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 48%[5][6] |
| Protein binding | 16%[6] |
| Metabolism | Liver (minimal; most excreted unchanged)[6] |
| Elimination half-life | 12 hours[5] |
| Excretion | Kidney[5] (23–46%),[7][8] Faecal[6] |
| Identifiers | |
| |
| Chemical and physical data | |
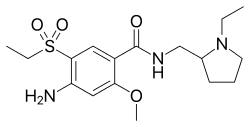
| Formula | C17H27N3O4S |
| Molar mass | 369.48 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Amisulpride, sold under the brand name Solian among others, is a medication used to treat and prevent postoperative nausea and vomiting (PONV) to treat schizophrenia.[3][9] It is taken by mouth or by injection into a vein.[3][9]
Common side effects include nausea, breast pain, sexual dysfunction, and low potassium.[3][9] Other side effects may include QT prolongation, high blood sugar, and abnormal lipids.[3][9] While its safety in pregnancy is unclear, it has caused harm with use in other animals.[10] It is an atypical antipsychotics and is believed to work by blocking the dopamine D2 and D3 receptors.[3]
Amisulpride has been in medical use since 1992.[11] It is available as a generic medication.[3] In the United Kingdom 60 pills of 400 mg costs about £42 as of 2021.[3] In the United States a vial of 5 mg costs about 45 USD.[12]
References edit
- ^ "Australian Product Information – Solian (Amisulpride) Tablets And Solution". TGA eBS. Archived from the original on 31 December 2019. Retrieved 10 May 2020.
- ^ a b "Amisulpride (Barhemsys) Use During Pregnancy". Drugs.com. 2 September 2020. Archived from the original on 28 January 2021. Retrieved 24 September 2020.
- ^ a b c d e f g h i j BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 415. ISBN 978-0857114105.
- ^ "Amisulpride 100 mg Tablets - Summary of Product Characteristics (SmPC)". (emc). 5 July 2019. Archived from the original on 26 February 2020. Retrieved 26 February 2020.
- ^ a b c Rosenzweig, P.; Canal, M.; Patat, A.; Bergougnan, L.; Zieleniuk, I.; Bianchetti, G. (2002). "A review of the pharmacokinetics, tolerability and pharmacodynamics of amisulpride in healthy volunteers". Human Psychopharmacology. 17 (1): 1–13. doi:10.1002/hup.320. PMID 12404702. S2CID 23877366.
- ^ a b c d "Solian tablets and solution product information" (PDF). TGA eBusiness Services. Sanofi-Aventis Australia Pty Ltd. 27 September 2019. Archived from the original on 31 December 2019. Retrieved 26 February 2020.
- ^ Caccia, S (May 2000). "Biotransformation of Post-Clozapine Antipsychotics Pharmacological Implications". Clinical Pharmacokinetics. 38 (5): 393–414. doi:10.2165/00003088-200038050-00002. PMID 10843459. S2CID 68853079.
- ^ Noble, S; Benfield, P (December 1999). "Amisulpride: A Review of its Clinical Potential in Dysthymia". CNS Drugs. 12 (6): 471–483. doi:10.2165/00023210-199912060-00005.
- ^ a b c d e f "Amisulpride Monograph for Professionals". Drugs.com. Retrieved 14 January 2022.
- ^ "Amisulpride (Barhemsys) Use During Pregnancy". Drugs.com. Archived from the original on 28 January 2021. Retrieved 14 January 2022.
- ^ Advances in the Neurochemistry and Neuropharmacology of Tourette Syndrome. Academic Press. 27 November 2013. p. 318. ISBN 978-0-12-411561-3. Archived from the original on 14 January 2022. Retrieved 14 January 2022.
- ^ "Barhemsys Prices, Coupons & Patient Assistance Programs". Drugs.com. Retrieved 14 January 2022.