 | This is a user sandbox of Jennaconnolly. You can use it for testing or practicing edits. This is not the sandbox where you should draft your assigned article for a dashboard.wikiedu.org course. To find the right sandbox for your assignment, visit your Dashboard course page and follow the Sandbox Draft link for your assigned article in the My Articles section. |
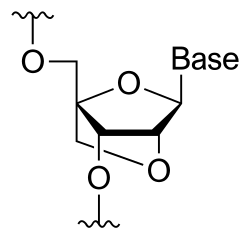
A locked nucleic acid (LNA), also known as bridged nucleic acid (BNA),[1] and often referred to as inaccessible RNA, is a modified RNA nucleotide in which the ribose moiety is modified with an extra bridge connecting the 2' oxygen and 4' carbon. The bridge "locks" the ribose in the 3'-endo (North) conformation, which is often found in the A-form duplexes. This structure can be attributed to the increased stability against enzymatic degradation;[2][3][4][5] moreover the structure of LNA has improved specificity and affinity as a monomer or a constituent of an oligonucleotide.[6] LNA nucleotides can be mixed with DNA or RNA residues in the oligonucleotide, in effect hybridizing with DNA or RNA according to Watson-Crick base-pairing rules.
Synthesis
editObika et al. were the first to chemically synthesize LNA in 1997[7], independently followed by Jesper Wengel's group in 1998.[8] This became possible after Zamecnick and Stephenson laid the groundwork on the possibility of oligonucleotides being great agents for controlling gene expression in 1978.[9] Till date, two different approaches, linear and convergent strategies respectively, have been shown to produce high yield and efficient LNAs. The linear strategy of synthesis was first detailed in the works of Obika et al.[7] In this approach, uridine (or any readily available RNA nucleoside) can be used as the starting material. The convergent strategy requires the synthesis of a sugar intermediate which serves a glycosyl donor necessary for coupling with nucleobases. Commonly, D-glucose is used to produce the sugar intermediate which is subsequently reacted with nucleobases using a modified Vorbrügen procedure allowing for stereoselective coupling.[10]
The addition of different moieties has remained a possibility with the maintenance of key physicochemical properties like the high affinity and specificity evident in the originally synthesized LNA.[8] Such oligomers are synthesized chemically and are commercially available.
LNAzymes are generally endonucleases that bind to specific RNA target sequences and cleave the phosphodiester bond that exists between the nucleotides.[11]They have become a prominent method for therapeutic and biotechnology applications due to their biostability compared to biological nucleic acids. Commonly referred to as LNAzymes, researchers have developed LNA modified oligonucleotides and have demonstrated remarkable hybridization to RNA, ssDNA, and dsDNA and facilitates mismatch repair in natural DNA[12]. Regarding the catalytic activity of LNAzymes, a more efficient cleavage of phosphodiester bonds in RNA substrates has been recorded as compared to DNAzymes.[13] Modification of the substrate recognition arms of DNAzymes with LNA monomers yields a LNAzyme which recognizes coxsackievirus A21 (CAV-21) and cleaves its RNA target sequence similar to one in the 5' untranslated region (5' UTR) of the human rhinovirus-14 (HRV-14); a sequence unrecognized by unmodified DNAzymes.[14]
Applications in Therapeutics and Biotechnology
editLNA-modified oligonucleotides is a promising option in the development of therapeutics due to its high stability in biological environments and preferential hybridization. Using LNA based oligonucleotides therapeutically is an emerging field in biotechnology.[15] A variety of LNA oligonucleotides have been assessed for their pharmacokinetic and toxicity profiles. The studies concluded that LNA toxicity is generally independent of oligonucleotide sequence, and displays a preferential safety profile for translatable therapeutic applications.[8] Allele-specific PCR using LNA allows for the design of shorter primers, without compromising binding specificity.[16] Additionally, LNA has been incorporated in fluorescence in situ hybridization (FISH).[17] FISH is a common technique used to visualize genetic material in a variety of cells, however previous studies note this technique has been limited by low probe hybridization efficiency. Conversely, LNA-incorporated probes demonstrated increased hybridization efficiency in both DNA and RNA. The improved efficiency of LNA-incorporated FISH has resulted in successful FISH analysis of the human chromosome, several types of non-human cells, and microarrays. LNA genotyping assays have been conducted as well, specifically to detect a mutation in apolipoprotein B[17]. LNA has been investigated for its therapeutic properties in treating cancers and infectious diseases. A novel locked nucleic acid phosphorothioate antisense molecule, termed SPC2996, has been developed to target the mRNA coding for Bcl-2 oncoprotein, a protein that inhibits apoptosis in chronic lymphocytic leukemia cells (CLL). Phase I and II clinical trials demonstrated a dose dependent reduction in circulating CLL cells in approximately 30% of the sample population, however, limitations and costs of this trial prompts further investigation into SPC2996.[18] LNA has also been applied to Miravirsen, an experimental therapeutic intended for the treatment of Hepatitis C, constituting a 15-nucleotide phosphorothioate sequence with binding specificity for MiR-122 (a miRNA expressed in hepatocytes).[19][20] Novel applications of LNA could enhance many forms of DNA and in effect be added to enzymes or drugs as a regulation mechanism. LNA has demonstrated promise in gene therapy for its potential to regulate gene expression but have shown mixed results in antisense studies. [15] Due to its high affinity for mismatch discrimination, LNA has been studied for its applications in diagnostic tools. Immobilized LNA probes have been successfully introduced in a multiplex SNP genotyping assay, an indication that LNA-incorporated diagnostics may emerge on the market in the future. [15]
- ^ Elayadi, Anissa N.; Braasch, Dwaine A.; Corey, David R. (2002-08). "Implications of High-Affinity Hybridization by Locked Nucleic Acid Oligomers for Inhibition of Human Telomerase †". Biochemistry. 41 (31): 9973–9981. doi:10.1021/bi025907j. ISSN 0006-2960.
{{cite journal}}: Check date values in:|date=(help) - ^ Kurreck, J. (2002-05-01). "Design of antisense oligonucleotides stabilized by locked nucleic acids". Nucleic Acids Research. 30 (9): 1911–1918. doi:10.1093/nar/30.9.1911. PMC 113840. PMID 11972327.
{{cite journal}}: CS1 maint: PMC format (link) - ^ Frieden, M. (2003-11-01). "Expanding the design horizon of antisense oligonucleotides with alpha-L-LNA". Nucleic Acids Research. 31 (21): 6365–6372. doi:10.1093/nar/gkg820. ISSN 1362-4962. PMC 275462. PMID 14576324.
{{cite journal}}: CS1 maint: PMC format (link) - ^ Frieden, Miriam; Hansen, Henrik F.; Koch, Troels (2003-10). "Nuclease Stability of LNA Oligonucleotides and LNA-DNA Chimeras". Nucleosides, Nucleotides and Nucleic Acids. 22 (5–8): 1041–1043. doi:10.1081/NCN-120022731. ISSN 1525-7770.
{{cite journal}}: Check date values in:|date=(help) - ^ Morita, K.; Hasegawa, C.; Kaneko, M.; Tsutsumi, S.; Sone, J.; Ishikawa, T.; Imanishi, T.; Koizumi, M. (2001-11-01). "2'-O, 4'-C-Ethylene-bridged nucleic acids (ENA) with nuclease-resistance and high affnity for RNA". Nucleic Acids Symposium Series. 1 (1): 241–242. doi:10.1093/nass/1.1.241. ISSN 0261-3166.
- ^ Veedu, Rakesh; Wengel, Jesper (2011). Medicinal Chemistry of Nucleic Acids. John Wiley & Sons, Inc. pp. 335–337. ISBN 0470596686.
- ^ a b Obika, Satoshi; Nanbu, Daishu; Hari, Yoshiyuki; Morio, Ken-ichiro; In, Yasuko; Ishida, Toshimasa; Imanishi, Takeshi (1997-12-15). "Synthesis of 2′-O,4′-C-methyleneuridine and -cytidine. Novel bicyclic nucleosides having a fixed C3, -endo sugar puckering". Tetrahedron Letters. 38 (50): 8735–8738. doi:10.1016/S0040-4039(97)10322-7. ISSN 0040-4039.
- ^ a b c Orum, Miriam Frieden and Henrik (2008-03-31). "Locked Nucleic Acid Holds Promise in the Treatment of Cancer". Current Pharmaceutical Design. doi:10.2174/138161208784246234. Retrieved 2020-10-06.
- ^ Zamecnik, P. C.; Stephenson, M. L. (1978-01-01). "Inhibition of Rous sarcoma virus replication and cell transformation by a specific oligodeoxynucleotide". Proceedings of the National Academy of Sciences. 75 (1): 280–284. doi:10.1073/pnas.75.1.280. ISSN 0027-8424. PMC 411230. PMID 75545.
{{cite journal}}: CS1 maint: PMC format (link) - ^ Koshkin, Alexei A.; Fensholdt, Jef; Pfundheller, Henrik M.; Lomholt, Christian (2001-12-01). "A Simplified and Efficient Route to 2'-O, 4'-C-Methylene-Linked Bicyclic Ribonucleosides (Locked Nucleic Acid)". The Journal of Organic Chemistry. 66 (25): 8504–8512. doi:10.1021/jo010732p. ISSN 0022-3263.
- ^ Breaker, R. R.; Joyce, G. F. (1994-12). "A DNA enzyme that cleaves RNA". Chemistry & Biology. 1 (4): 223–229. doi:10.1016/1074-5521(94)90014-0. ISSN 1074-5521. PMID 9383394.
{{cite journal}}: Check date values in:|date=(help) - ^ Veedu, Rakesh N.; Vester, Birte; Wengel, Jesper (2007-03-26). "Enzymatic Incorporation of LNA Nucleotides into DNA Strands". ChemBioChem. 8 (5): 490–492. doi:10.1002/cbic.200600501.
- ^ Vester, Birte; Lundberg, Lars Bo; Sørensen, Mads D.; Babu, B. Ravindra; Douthwaite, Stephen; Wengel, Jesper (2002-11). "LNAzymes: Incorporation of LNA-Type Monomers into DNAzymes Markedly Increases RNA Cleavage". Journal of the American Chemical Society. 124 (46): 13682–13683. doi:10.1021/ja0276220. ISSN 0002-7863.
{{cite journal}}: Check date values in:|date=(help) - ^ Schubert, Steffen; Fürste, Jens P; Werk, Denise; Grunert, Hans-Peter; Zeichhardt, Heinz; Erdmann, Volker A; Kurreck, Jens (2004-05). "Gaining Target Access for Deoxyribozymes". Journal of Molecular Biology. 339 (2): 355–363. doi:10.1016/j.jmb.2004.03.064.
{{cite journal}}: Check date values in:|date=(help) - ^ a b c Petersen M, Wengel J (February 2003). "LNA: a versatile tool for therapeutics and genomics". Trends in Biotechnology. 21 (2): 74–81. doi:10.1016/S0167-7799(02)00038-0. PMID 12573856.
- ^ Bonetta L (2005). "Prime time for real-time PCR". Nat. Methods. 2 (4): 305–312. doi:10.1038/nmeth0405-305.
- ^ a b Kubota, Kengo; Ohashi, Akiyoshi; Imachi, Hiroyuki; Harada, Hideki (2006-08). "Improved in situ hybridization efficiency with locked-nucleic-acid-incorporated DNA probes". Applied and Environmental Microbiology. 72 (8): 5311–5317. doi:10.1128/AEM.03039-05. ISSN 0099-2240. PMC 1538721. PMID 16885281.
{{cite journal}}: Check date values in:|date=(help) - ^ Dürig, J.; Dührsen, U.; Klein-Hitpass, L.; Worm, J.; Hansen, J. B. Rode; Ørum, H.; Wissenbach, M. (2011-04). "The novel antisense Bcl-2 inhibitor SPC2996 causes rapid leukemic cell clearance and immune activation in chronic lymphocytic leukemia". Leukemia. 25 (4): 638–647. doi:10.1038/leu.2010.322. ISSN 1476-5551.
{{cite journal}}: Check date values in:|date=(help) - ^ Gebert, Luca F. R.; Rebhan, Mario A. E.; Crivelli, Silvia E. M.; Denzler, Rémy; Stoffel, Markus; Hall, Jonathan (2014-01-01). "Miravirsen (SPC3649) can inhibit the biogenesis of miR-122". Nucleic Acids Research. 42 (1): 609–621. doi:10.1093/nar/gkt852. ISSN 0305-1048. PMC 3874169. PMID 24068553.
- ^ Bonneau, E.; Neveu, B.; Kostantin, E.; Tsongalis, G.J.; De Guire, V. (2019-06-24). "How close are miRNAs from clinical practice? A perspective on the diagnostic and therapeutic market". EJIFCC. 30 (2): 114–127. ISSN 1650-3414. PMC 6599191. PMID 31263388.