Silsesquioxanes
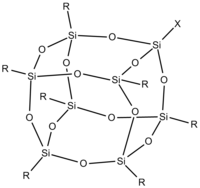
A silsesquioxane is a compound with the empirical chemical formula RSiO3/2 where Si is the element silicon, O is oxygen and R is either hydrogen or an alkyl, alkene, aryl, arylene group[1]. This materials can be used as a support for catalysts[2]. Silsesquioxanes can have a cage like structure, see Figure 1, which is most commonly cubes, hexagonal prisms and octagonal prisms[3].
Background
editSince their initial discovery, silsesquioxanes have been a frequent and productive topic of research that has become interwoven into many fields of science, including energy, materials, catalysis and bioengineering[4]. The extent of this variation is largely due to the molecules themselves, which have been found to form a number of different structure types. Though the basic formula for all silsesquioxanes has been found to be RSiO3/2 with the identity of R typically being alkyl or organo-functional groups, the combined structure of these RSiO3/2 units vary depending on synthesis methods, starting materials and the catalyst used. The four most common silsesquioxane structures are cage structures in which the units form a cage of n units in a designated Tn cage, partial cage structures, seen in Figure 2, in which the aforementioned cages[5] are formed but lack complete connection of all units in the cage, ladder structures in which two long chains composed of RSiO3/2 units are connected at regular intervals by Si-O-Si bonds, and finally random structures which include RSiO3/2 unit connections without any organized structure formation[4].
The high three dimensional symmetry and nanometer size makes silsesquioxanes very useful building blocks for nanocomposites. The diversity of possible functional groups along with their controlled orientation in 3-D space allows for highly tailored nanometer-by-nanometer construction in all three dimensions with these unique nanobuilding blocks. The silica core gives rigidity and thermal stability that provides mechanical and thermal properties surpassing typical organics. Combining the robust core with the functionalities of the attached organic groups can also change the physical properties of the SQs allowing for easier processing than typical ceramics. The mixture of organic and inorganic functionalities can lead to the creation of novel nanocomposite materials that exhibit properties intermediate and superior to those of traditional polymer and ceramic properties. Tailored SQs provide a solution where processing conditions prevent ceramic-like materials from being plausible or mechanical requirements prevent polymer-like materials from being useful. The significance of SQs can be seen from their vast application possibilities in diverse fields including aerospace, antimicrobials, photonics, microelectronics, semiconductors, cosmetics and catalysis science.
In recent years, silsesquioxanes have become commonly known as polyhedral oligosilsesquioxanes (POSS), which is a trademarked name by Hybrid Plastics. Hybrid Plastics was formed by Dr. Joseph Lichtenhan and his colleagues in 1998 and since has been a large catalyst in expanding the field. This is largely due to Hybrid Plastics making many silsesquioxane feedstocks commercially available.
Mayaterials, Inc., a research and development company founded in 2003, also produces octasilsesquioxanes of the type [ROSiO1.5]8 and its polymeric derivatives, and [RphenylSiO1.5]8 and its polymeric derivatives. In addition to selling a wide array silsesquioxanes, they use these nanobuilding blocks to make nanocomposites for thin films, monoliths, and fiber reinforced composites. In 2009, Mayaterials spun-out EXIMO Nanoengineered Coatings to commercialize its silsesquioxane coatings as semi-permanent mold release for the tooling industry.
Chemical Structure and Synthesis
editThe structure of silsesquioxanes depends on the preparation method[6]. To simplify, a silicon with a hydrolytically stable organic substituent and three easily hydrolyzed groups such as chlorine or alkoxy groups, which are reacted with water and an acid or base catalyst. The final structure depends on the function of the concentration of initial monomer, concentration of water, temperature, type of catalyst, and the nature of the non-hydrolyzing substituent. This can be seen in the following equations[6]. Solvent hydrogen bonding can have a large effect on rates and types of molecular condensations.
The basic silsesquioxane synthesis methods introduced by those who pioneered its formation in the silicone industry typically involved producing trichlorosilane precursors. These precursors were often formed by the reaction of methylene chloride or hydrogen chloride with silicon metal in the presence of a metal catalyst. The subsequent reaction used to form the silsesquioxanes were typically metal-catalyzed hydrosilylation reactions with chloro- or alkylsilanes or organometallic coupling reactions with chlorosilanes. The choice of metal catalyst is dependent on the selected R substituents to be attached (for instance, with the addition of alkyl groups larger than methyl or organo-functional groups, platinum is used as a catalyst). Some work has been done for limited alterations of substituent addition, but in general there is to date no known method to direct or control the formation of particular substitutional isomers when introducing two or more different substituents in a silsesquioxane cage synthesis.
When characterizing silsesquioxanes the typical methods involved are x-ray diffraction, nuclear magnetic resonance spectroscopy (proton, carbon and silicon), and infrared radiation spectroscopy, though SEM and TEM have been used for visualization in crystal growth studies. Features of general importance when describing silsesquioxane compounds are the number of RSiO3/2 units in the cage compounds and the degree to which the silsesquioxane is condensed. In a fully condensed silsesquioxane compound, the general formula is RaSiaO(1.5a–0.5b)(OH)b with b=0, indicating that all oxygen atoms in the compound are bridging silicon atoms. A less condensed silsesquioxane compound (b>0) is indicative of the compound having less coordinated Si-O-Si connections, thus in general how condensed the compound is gives the nature of the macroscopic molecule (i.e. polymeric forms are typically highly condensed- almost fully connected networks). It is worth noting that when the cage formations of silsesquioxanes are produced, though many different sizes are possible (i.e. T8, T10, T12), the most preferred formation are the cubic T8 compounds due to the high stability of the Si4O4 rings in the cage.
Many polymeric forms of silsesquioxanes have been developed with varying molecular weights and synthesis methods. The first high molecular weight tractable polymeric silsesquioxane was a ladder type repeating unit, seen in Figure 3, polyphenylsilsequioxane, reported by Brown et al. in 1960[7]. Brown’s findings were used as a basis for further research and synthesis variations by plethora of additional research groups investigating polyphenylsilsequioxanes. Though many alterations were made, the origin synthesis proposed by Brown involved a three step process outlined as follows: (1) the hydrolysis of phenyltrichlorosilane in a solvent with excess water to give a hydrolyzate, (2) equilibration of the hydrolyzate with potassium hydroxide at a low concentration and temperature to give the prepolymer, and (3) equilibration of the prepolymer at a high concentration and temperature to give the final polymeric form. It was found that the critical factors to increase the polymer weight were high concentration and temperature during the equilibration of the prepolymer. Another noteworthy milestone in the silsesquioxane polymeric materials is the development of soluble and stable polymethylsilsesquioxane with high molecular weights by Japan Synthetic Rubber[8]. This polymer which, unlike its phenyl derivative, gels easily during the course of its synthesis, has found a wide range of alternative applications including cosmetics[9], resins[10], and chemical amplification resist for electron beam lithography[11].
Another scientific development in the field of silsequioxanes was the first synthesis of hydridosilsesquioxanes by Frye and Collins[12]. Hydridosilsesquioxanes are a silsesquioxane type with only hydrogen substituents on the silicon, and are thus purely inorganic compounds. Initial synthesis methods involved the adding benzene solutions of trichlorosilane to a mixture of benzene, concentrated sulfuric acid, and fuming sulfuric acid to yield the T10-T16 oligomers. The T8 oligomer was also synthesized, but by the reaction of trimethylsilane with a mixture of acetic acid, cyclohexane, and hydrochloric acid. It has been found that these compounds can be converted to silica coatings for application in environmental protection, and for application as an interlayer dielectric for integrated circuits[13][14].
Silsesquioxanes for Electronics
editThe synthesis of silsesquioxane materials for electronics applications can be quite detailed, with many variations occurring through the desired shapes of these structures as well as the different organic groups attached to these structures.
The bridged polysilsesquioxanes were developed originally to produce controlled porosity in structures[6]. Bridging refers to structures where two or more –SiO(3/2) units are attached by the same organic fragment to form molecular composites[6]. These are most readily prepared from molecular building blocks that contain two or more trifunctional silyl groups attached to non-hydrolysable silicon-carbon bonds, with typical sol-gel processing. Monomers are usually dissolved in miscible solvent of water, with hydrolysis and condensation reactions catalyzed by acid, base or fluoride. The catalyst changes the physical properties of the silsesquioxane structures. Acid catalysts give clear, brittle solids, and base catalysts give opaque solids. It was found that mesopore size is proportional with the length of the bridge.
Synthesis of organosilsesquioxane films for semiconducting devices can be summarized as follows. A trichlorosilane is added drop-wise to distilled water and some non-polar solvent such as hexanes at 0°C[15]. Reaction left to stir for some time to allow precipitate to form, which is then filtered. Hexane is then added to the aqueous reaction medium to extract the product. This general reaction gives a basic synthesis for hydrogen silsesquioxanes. These reactions often use platinum catalysts such as chloroplatinic acid to get desired properties. Commercially available silsesquioxanes can then be modified to alkylated silsesquioxanes by the cross-metathesis of alkenes with readily available vinyl-substituted silsesquioxanes. In order to form a low k dielectric film, copolymers of alkylsilanes are copolymerized with trichlorosilane, with properties being controlled by the ratios of each[15]. These polymers are then separated by molecular weight, since only low molecular weight polymers can be applied by Chemical Vapor Deposition (CVD) to a device. This is usually obtained by heating above the vapor pressure in a vacuum. There are also many other methods of applying these thin films for semiconductor devices such as spin coating, dip-coating, and spraying. The resulting material would have a molecular formula of [R-SiO1.5]x[H-SiO1.5]y with x+y being an integer between 5 and 30. The methods described for forming thin films are useful in filling in empty space in electric materials as well as giving them an even surface. There has also been interest in applying caged silsesquioxanes to these materials[16]. Poly(methylsilsesquioxane), as mentioned above is an example of such a species. These materials give cage structures of varying sizes that are controlled by the synthetic processing. In general hydrolyzing hydrido- or organo- trichlorosilanes forms cages. Temperatures are below room temperature, and the system is kept dilute to favor intramolecular condensations. Condensation rates have also been found to slow by hydrogen bonding solvents. In general, caged structures are formed by kinetic not thermodynamic control.
In the application of light emitting diodes, there have been many more recent advances in synthetic techniques and functionalization of cubic silsesquioxanes[4]. One of the first precursors used in light emitting application was octadimethylsiloxysilsesquioxane, which can be prepared in yields of >90% by treating tetraethoxysilane or rice hull ash with tetramethylammonium hydroxide followed by dimethylchlorosilane. The general method of hydrolyzing organotrichlorosilanes is still effective here. Recent research is looking at the effects of phenyl(silsesquioxane) structures, which can be functionalized to have a light emitting component from the organic end, through aromatic substitution reactions. When brominated or aminated, these structures can be coupled with epoxies, aldehydes, and bromoaromatics. The main goal is to attach these silsesquioxanes to π-conjugated polymer systems. Which can be done through the same functionalization methods mentioned above. These methods can use copolymerization techniques, Grignard reagents, and different coupling strategies. There has also been research on the ability of conjugated dendrimer silsesquioxanes to behave as light emitting materials. Though, highly branched substituents tend to have π-π interactions, which hinder high luminescent quantum yield.
It has been demonstrated by many research groups that chemically incorporating silsesquioxanes, can improve materials properties such as solubility, amorphousness, thermal and oxidative stability. This in turn leads to improved OLED device efficiencies and lifetimes. Whether the strategy involves linking the silsesquioxane cage to a polymer backbone to minimize aggregation, or linking active moieties to the rigid silsesquioxane core to form amorphous materials, it is clear that improved properties can be achieved.
Applications for Electronics
editExtensive research on silsesquioxanes as semiconductors, insulators and organic light emitting diodes (OLED) has been done by many companies and Universities, including Dow Corning, IBM, Honeywell, Japan Rubber Co, Hitachi, Mayaterials, Hybrid Plastics, University of Michigan and University of California-Irvine. These materials can be used in semiconductor devices as both semiconducting materials, with tuned functionality resulting from the attached organic groups, or as insulators in their native forms to form spacing in layers in semiconducting devices[17][15]. These materials tend to have low dielectric constants (k), which makes them good thin film insulators[15]. As organic OLEDs, polyhedral oligomeric silsesquioxanes make up an inorganic core with peripheral organic emitters making up the peripheral of a complex. This incorporation allows for an improved stability and an enhancement in electroluminescence properties.
The first example of a type of silsesquioxane that has found application for interlayer dielectric applications is poly(hydridosilsesquioxane), which represents a linked-cage structure, which is sold under the name Fox Flowable Oxide. These hydrogen silsesquioxanes are readily used for ceramic coatings in devices such as semiconductors and can be found not only in the linked-cage (see Figure 5), but also in the ladder form as well (see Figure 4). These compounds are often applied to an electronic device with organic solvent through evaporative techniques for thin-film coating[15]. These devices can be difficult to prepare due to the fact that silsesquioxanes can be unstable in solvents and it is difficult to control film thickness.
Methylsilsesquioxane materials are useful as spin-on-glass (SOG) dielectrics. Bridged silsesquioxanes have been used for quantum confined nano-size semiconductors. Silsesquioxane resins have also been used for these applications because they have high dielectric strengths, low dielectric constants, high volume resistivities, and low dissipation factors, making them very suitable for electronics applications. These resins have heat and fire resistant properties, which can be used to make fiber-reinforced composites for electrical laminates.
With electronics getting smaller and smaller, the need for materials that keep these devices from short-circuiting is growing in demand[15]. Single microchips contain thousands of interconnecting transistors that when overlapped, can cause interference problems, power dissipation and voltage issues. Silsesquioxane properties have the ability to prevent short-circuiting by acting as a rigid, insulating spacer; preventing corrosion or oxidation of metal conductors. They can level uneven topography, and fill gaps between closely spaced conductors. These films are easily applied through solvent evaporation with hydrogen silsesquioxane resins, and turned ceramic by heating the substrate in air. These films are known as interlevel dielectric (ILD) and protective overcoat films (PO).
Industrial applications of OLEDs have a limited application. Traditional OLEDs do not typically contain inorganic materials, however due to the instability of OLEDs on their own, research is being conducted to look at hybrid materials that increase the stability of these compounds. Polyhedral oligomeric silsesquioxanes have been looked at in order to form an inorganic core. These compounds give better mechanical properties and stability, with an organic matrix for good optical and electrical properties[18]. The mechanisms of degradation in these devices is not well understood, but it is believed that material defect understanding is important for understanding the optical and electronic properties.
Silsesquioxane Catalysis
editAnother area involving silsesquioxanes that has experienced increased scientific development recently is the study of metal coordination of silsesquioxanes, resulting in metallasilsesquioxanes, which have found application as catalysts. Incompletely condensed silsesquioxanes like Cy7Si7O9(OH)3 are similar in structure to β-tridymite and β-cristobalite, making them good models for the silanol sites on silica surfaces[19]. The structures of silsesquioxanes make them ideal for metal coordination as well, due to the fixed orientation of the silanol groups and also the siloxane bridges which can interact with the metal. Additionally the silsesquioxane can be modified for even better metal coordination via simply silylation reactions[20]. Research has shown that silsesquioxanes can bind with numerous main group and transition group metals, including Na, Li, and Be[21][22][19]. The most frequently employed starting silsesquioxane for metal complex synthesis is the trisilanol derivative Cy7Si7O9(OH)3, originally reported by Brown and Vogt, which is synthesized from trichlorocyclohexylsilane, but can take several years to run to completion[7]. This resulted in only a few incompletely condensed silsesquioxanes available in useful quantities for research. Consequently most research has been focused on the trisilanol derivative Cy7Si7O9(OH)3and its cyclopentyl-substitued analog. However recently Feher et al. have developed an acid-mediated cleavage of fully condensed silsesquioxane frameworks like Cy8Si8O12[23]. The process results in silanediols that can further be used to create new metallasilsesquioxanes.
The general preparation for metal-silsesquioxane derivatives involves treating the parent silanol and the desired metal halide in the presence of a base like triethylamine[24]. The product metallasilsesquioxane can frequently be readily isolated by fractional crystallization. Another route of synthesis involves first deprotonating the trisilanol group, however this has proven to be somewhat difficult. Initial attempts by Feher et al. to deprotonate trisilanols with sodium t-butoxide did so, but the products were unstable for an extended period of time. More recently it was found that deprotonated trisilanols could successfully be prepared if the right base was used. Feher et al. determined three equivalents of LiN(SiMe3)2 were effective, with the product potentially even precipitating out depending on the solvent[25]. Aspinall et al. later succeeded in doing the same using three equivalents of n-BuLi in hexanes and further results indicate that alkali metal derivatives of deprotonated silsesquioxanes could also be prepared using alkali metalbis(trimethylsilyl) amides[26].
Much of modern research is focused on the synthesis of metallasilsesquioxanes that containing metals that have not been done before and the potential application of the metallasilsesquioxanes as catalysts, as it has become accepted that metallasilsesquioxanes are good silica-supported transition metal catalysts. For example Edelmann et al. successfully synthesized and analyzed the first beryllium silsesquioxane, [Cy7Si7SO12BeLi]2.2THF[19]. The use of silsesquioxanes to make homogeneous models for heterogeneous catalysts is another area research, allowing a better understanding of the system to be reached.
Applications in catalysis
editMetallasilsesquioxanes have found wide use as catalysts for both homogeneous and heterogeneous systems. The complexes have found application as catalysts for alkene metathesis, polymerization, epoxidation and Diels-Alder reactions of enones. A number of metallasilsesquioxanes have been reported that can polymerize ethene, which generally contain chromium as the metal, due to its well-known use in industry in the Phillips catalyst[27]. The catalyst can be easily activated with trimethylaluminum and typically proceeds for several hundred to more than 3200 turnovers[27]. Vanadium complexes as well as Ziegler–Natta type catalysts have also been employed as ethene polymerization catalysts[28]. The coordination of metals to the silsesquioxane framework gives electrophilic centers that are approximately as electron-withdrawing as a CF3 group, leading to increased catalytic activity[29]. Complexes of silsesquioxane and molybdenum and tungsten have been reported as alkene metathesis catalysts. The metallasilsesquioxanes complex with tungsten has even been recently used for ring-opening metathesis polymerization of norborene[29]. The epoxidation of alkenes by the metallasilsesquioxanes is currently an area of high interest, with many groups focused on its development. Crocker et al. have reported catalysts for alkene epoxidation that use peroxide. Abbenhius et al. have recently reported using silsesquioxanes for the construction of polyoxometalaltes as well, which have potential for use in oxidation processes that currently use environmentally friendly oxidizing agents like O2 and H2O2[29]. The first reported catalytic alkene epoxidation was done by a titanium complex, epoxidizing compounds like cyclooctene and norbornene nearly quantitatively. Further research has revealed the silsesquioxane-based complexes can promote Diels-Alder reactions, as well as other Lewis acid catalyzed reactions like Oppenauer oxidation and Meerwein-Pondorf-Verley reductions. The application of silsesquioxanes as catalysts as barely begun and with easier methods for syntheses of the complexes being published further applications can be anticipated.
Antimicrobial Silsesquioxanes
editIn recent research efforts silsesquioxanes have been functionalized with biocidal groups to produce antimicrobial coatings. Such coatings have numerous applications, as there is a continually increasing demand for materials that will keep people safe and healthy. Organic ammonium salts are well-known for their antimicrobial properties, meaning their ability to kill or inhibit the growth of harmful microbes. Specifically, quaternary ammonium salts (QAS) are used as disinfectants, antiseptics, and antifoulants that kill bacteria, fungi, and algae, but are not harmful to humans and animals[30][31]. Several research groups have functionalized polyhedral oligomeric silsesquioxanes with QASs. The relatively small size of the silsesquioxane molecule, 2-5 nm, allows a QAS functionalized molecule to have a charge density similar to dendrimers and thus the antimicrobial efficacy is prominent. Chojnowski et al. explored the quaternization of dimethyl-n-octylamine by octa(3-chloropropylsilsesquioxane), (T-ClPr)8, and transformed almost all eight chloropropyl groups into ionic quaternary ammonium chloride functions[32]. The synthesis is based on a three-step hydrolytic polycondensation process of 3-chlo- ropropyltrimethoxysilane. The resulting material exhibited antimicrobial efficacy for the prevention of growth of both Gram-positive and Gram-negative bacteria.
More recently, Majumdar et al. synthesized an array of QAS functionalized polyhedral oligomeric silsesquioxanes (Q-POSS)[33]. These researchers varied the alkyl chain length from –C12H25 to –C18H37 and varied the counter ion between chloride, bromide, and iodine. The first reaction was the hydrosilylation between allydimethlamine and octasilane polyhedral oliomeric silsesquioxane via Karstedt’s catalyst to make a tertiaryamino-functinoalized silsesquioxane. The second step was the quaternization of the tertiaryamino groups with an alkyl halide. The alkyl halides used were 1-iodooctadecane, 1-bromohexadecane, and 1-chloroctadecane.
Applications of Antimicrobial Silsesquioxanes
editQuaternary ammonium-functionalized polyhedral olgiomeric silsesquioxanes are useful for antimicrobial applications. The silsesquioxane core in such hybrid materials provides an increased glass transition temperature, improved mechanical properties, higher use temperature, and lower flammability. These desirable properties combined with the ability to readily functionalize a silsesquioxane with multiple antimicrobial groups allows for robust biocides with higher charge densities while maintaining a compact molecular structure. The organic functionalities provide high compatibility with polymers allowing for easy incorporation into many mediums. Of particular interest are silicone paints and coatings used in hospitals. Typical biocidal ammonium functionalized polymers are incompatible, but silsesquioxanes closely mimic the silicone structure. A silicone based paint combined with QAS-functinalized silsesquioxanes could be used to paint medical and sanitary devices, biomedical devices, exam equipment, medical storage rooms, hospital rooms, clinics, doctor offices, etc. to prevent the formation and spread of bacteria. For example, the Q-POSS developed by Majumbar et al. was combined with polydimethylsiloxane and catalysis to form a crosslinked network[33]. The researchers found that coatings based on bromide and chloride had the best antimicrobial efficacy.
References
edit- ^ Guizhi Li, Lichang Wang; Pittman Jr, Charles U. (2001). "Polyhedral Oligomeric Silsesquioxane (POSS) : a Review". Journal of Inorganic and Organometallic Polymers. 11: 123. doi:10.1023/A:1015287910502.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Layer-by-layer assembly of Ru3+ and Si8O208- into electrochemically active silicate films, Rassaei, L; Sillanpaa, M; Milsom, EV, et al.,Journal of solid state electrochemistry 12 (2008) 747-755.
- ^ Chem. Rev. 2010. doi:10.1021/cr900201r.
{{cite journal}}: Missing or empty|title=(help) - ^ a b c Chan, K. L.; Sonar, P.; Sellinger, A. (2009). Journal of Materials Chemistry. 19: 9103.
{{cite journal}}: Missing or empty|title=(help)CS1 maint: multiple names: authors list (link) - ^ Baney, R.H.; Itoh, M.; Sakakibara, A.; Suzuki, T. (1995). Chem. Rev. 95: 1409-1430.
{{cite journal}}: Missing or empty|title=(help)CS1 maint: multiple names: authors list (link) - ^ a b c d Jones, R. G.; Ando, W.; Chojnowski, J. (2000). (1st ed.). Dodrecht, The Netherlands: Kluwer Academic Publishers. p. 157-183.
{{cite book}}: Missing or empty|title=(help); Unknown parameter|book=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b Brown, J. F., Jr.; Vogt, J. H., Jr.; Katchman, A.;'Eustance, J.W.; Kiser, K. M.; Krantz, K. W. (1960). J. Am. Chem. Soc. 82: 6194. doi:10.1021/ja01508a054.
{{cite journal}}: Missing or empty|title=(help)CS1 maint: multiple names: authors list (link) Cite error: The named reference "Brown" was defined multiple times with different content (see the help page). - ^ Suminoe. T.: Matsumura. Y.: Tomomitsu. 0. (1978). Chem. Abstr. 89: 180824.
{{cite journal}}: Missing or empty|title=(help)CS1 maint: numeric names: authors list (link) - ^ Hase, N.; Tokunaga, T. (1993). Chem. Abstr. 119: 34107.
{{cite journal}}: Missing or empty|title=(help)CS1 maint: multiple names: authors list (link) - ^ Dote, T.; Ishiguro, K.; Ohtaki, M.; Shinbo, Y. (1990). Chem. Abstr. 113: 213397.
{{cite journal}}: Missing or empty|title=(help)CS1 maint: multiple names: authors list (link) - ^ Watanabe, H.; Todokoro, Y.; Inoue, M. (1991). Microelectron Eng. 13: 69.
{{cite journal}}: Missing or empty|title=(help)CS1 maint: multiple names: authors list (link) - ^ Frye, C. L.; Collins, W. T. (1970). J. Am. Chem. Soc. 92: 5586. doi:10.1021/ja00722a009.
{{cite journal}}: Missing or empty|title=(help)CS1 maint: multiple names: authors list (link) - ^ Gentle, T.E. (1991). Proc. SHE-Int. Soc. 1595: 146.
{{cite journal}}: Missing or empty|title=(help) - ^ Chandra, G. (1991). Mater. Res. Soc. Symp. Proc. 203: 97.
{{cite journal}}: Missing or empty|title=(help) - ^ a b c d e f Hacker, N.P. (2002). US Pat. 6472076: 7-9.
{{cite journal}}: Missing or empty|title=(help) - ^ Voronkov, M.G.; V.I. Lavrent’yev, V.I. (1982). Top. Curr. Chem. 102: 199-236.
{{cite journal}}: Missing or empty|title=(help)CS1 maint: multiple names: authors list (link) - ^ Nozue, I. (1986). US. Pat. 4626556: 6.
{{cite journal}}: Missing or empty|title=(help) - ^ Renaud, C.; Josse, Y.; Lee, C.-W.; Nguyen, T.-P. (2008). Journal of Materials Science: Materials in Electronics. 19: 87-91.
{{cite journal}}: Missing or empty|title=(help)CS1 maint: multiple names: authors list (link) - ^ a b c Lorenz, V.; Fischer, A.; Edelmann, F. T. (2000). Inorg. Chem. Comm. 3: 292-295. doi:10.1016/S1387-7003(00)00071-X.
{{cite journal}}: Missing or empty|title=(help)CS1 maint: multiple names: authors list (link) - ^ Dijkstra,T.W.; Duchateau,R.; Van Santen,R.A.; Meetsma,A.; Yap,G.P.A. (2002). J. Am. Chem. Soc. 124: 9856-9864. doi:DOI: 10.1021/ja0122243.
{{cite journal}}: Check|doi=value (help); Missing or empty|title=(help)CS1 maint: multiple names: authors list (link) - ^ Feher,F.J.; Budzichowski,T.A. (1995). Polyhedron. 14: 3239-3253. doi:10.1016/0277-5387(95)85009-0.
{{cite journal}}: Missing or empty|title=(help)CS1 maint: multiple names: authors list (link) - ^ Duchateau,R.; Gerritsen,G.; Van Santen,R.A.; Yap,G.P. (2003). Organometallics. 22: 100-110. doi:10.1021/om0200858.
{{cite journal}}: Missing or empty|title=(help)CS1 maint: multiple names: authors list (link) - ^ Feher, F.J.; Soulivong, D.; Nguyen, F. (1988). Chem. Commun. 12: 1279-1280. doi:10.1039/A802670J.
{{cite journal}}: Missing or empty|title=(help)CS1 maint: multiple names: authors list (link) - ^ Murugavel, R.; Voigt, A.; Walawalkar, M.G; Roesky, H.W. (1996). Chem. Rev. 96: 2205-2236. doi:10.1021/cr9500747.
{{cite journal}}: Missing or empty|title=(help)CS1 maint: multiple names: authors list (link) - ^ Feher, F.J.; Rahimian, K.; Budzichowski, T.A.; Ziller, J.W. (1995). Organometallics. 14: 3920-3926. doi:10.1021/om00008a043.
{{cite journal}}: Missing or empty|title=(help)CS1 maint: multiple names: authors list (link) - ^ Lorenz,V.; Fischer,A.; Edelmann,F.T. (2000). Coord. Chem. Rev. 206: 321-368. doi:10.1016/S0010-8545(00)00299-X.
{{cite journal}}: Missing or empty|title=(help)CS1 maint: multiple names: authors list (link) - ^ a b Abbenhuis, H.C. (2000). Chem. Eur. J. 6: 25-32. doi:10.1002/(SICI)1521-3765(20000103)6:1<25::AID-CHEM25>3.0.CO;2-Y.
{{cite journal}}: Missing or empty|title=(help) - ^ Karol, F.J. (1972). J. Poly. Sci. Part A-1. 10: 2621-2637. doi:10.1002/pol.1972.150100910.
{{cite journal}}: Missing or empty|title=(help) - ^ a b c Feher, F. J.; Tajima, T. L. (1994). J. Am. Chem. Soc. 116: 2145-2146. doi:10.1021/ja00084a065.
{{cite journal}}: Missing or empty|title=(help)CS1 maint: multiple names: authors list (link) - ^ Russel, A.D. (1969). Prog. Med. Chem. 6: 135-199.
{{cite journal}}: Missing or empty|title=(help) - ^ Sauvet, G.; Fortuniak, W.; Kazmierski, K.; Chojnowski, J. (2003). J. Polym. Sci. A: Polym. Chem. 41: 2939-2948.
{{cite journal}}: Missing or empty|title=(help)CS1 maint: multiple names: authors list (link) - ^ Chojnowski, J.; Fortuniak, W.; Rosciszewski, P.; Werel, W.; Łukasiak, J.; Kamysz, W.; Hałasa, R. (2006). J. Inorgan. Organomet. Polym. Mater. 16: 219-230. doi:10.1007/s10904-006-9048-5.
{{cite journal}}: Missing or empty|title=(help)CS1 maint: multiple names: authors list (link) - ^ a b Majumdar, P.; He, J.; Lee, E.; Kallam, A.; Gubbins, N.; Stafslien, S.J.; Daniels, J.; Chishom, B.J. (2010). J. Coat. Technol. Res. 7: 455-467. doi:10.1007/s11998-009-9197-x.
{{cite journal}}: Missing or empty|title=(help)CS1 maint: multiple names: authors list (link)