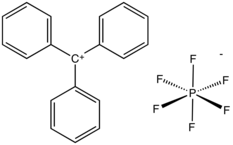
Triphenylmethyl hexafluorophosphate (also triphenylcarbenium hexafluorophosphate, trityl hexafluorophosphate, or tritylium hexafluorophosphate) is an organic salt with the formula [(C6H5)3C]+[PF6]−, consisting of the triphenylcarbenium cation [(C6H5)3C]+ and the hexafluorophosphate anion [PF6]−.[1]
| |

| |
| |
| Names | |
|---|---|
| IUPAC name
Triphenylcarbenium hexafluorophosphate
| |
| Systematic IUPAC name
Diphenylmethylbenzene hexafluoridophosphate | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.006.467 |
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| [(C6H5)3C]+[PF6]− | |
| Molar mass | 388.293 g·mol−1 |
| Appearance | Brown powder |
| Melting point | 145 °C (293 °F; 418 K) |
| Hazards | |
| GHS labelling: | |

| |
| Danger | |
| H314 | |
| P260, P264, P280, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P321, P363, P405, P501 | |
| Safety data sheet (SDS) | [1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Triphenylmethyl hexafluorophosphate is a brown powder that hydrolyzes readily to triphenylmethanol. It is used as a catalyst and reagent in organic syntheses.[2]
Preparation
editTriphenylmethyl hexafluorophosphate can be prepared by combining silver hexafluorophosphate with triphenylmethyl chloride:[3]
- Ag+[PF6]− + (C6H5)3CCl → [(C6H5)3C]+[PF6]− + AgCl
A second method involves protonolysis of triphenylmethanol:[4]
- H[PF6] + (C6H5)3COH → [(C6H5)3C]+[PF6]− + H2O
Structure and reactions
editTriphenylmethyl hexafluorophosphate readily hydrolyzes, in a reaction that is the reverse of one of its syntheses:[5]
- [(C6H5)3C]+[PF6]− + H2O → (C6H5)3COH + H[PF6]
Triphenylmethyl hexafluorophosphate has been used for abstracting hydride (H−
) from organic compounds. Treatment of metal-alkene and diene complexes one can generate allyl and pentadienyl complexes, respectively.[2]
Triphenylmethyl perchlorate is a common substitute for triphenylmethyl hexafluorophosphate. However, the perchlorate is not used as widely, because, like other organic perchlorates, it is potentially explosive.[2]
See also
editReferences
edit- ^ Triphenylcarbenium hexafluorophosphate from PubChem
- ^ a b c Urch, C. (2001). "Triphenylmethyl Hexafluorophosphate". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rt363f. ISBN 0471936235.
- ^ Sharp, D. W. A.; Sheppard, N. (1957). "Complex Fluorides. Part VIII". Journal of the Chemical Society (Resumed): 674–682. doi:10.1039/JR9570000674.
- ^ Olah, G.; Svoboda, J.; Olah, J. (1972). "Preparative Carbocation Chemistry; IV. Improved Preparation of Triphenylcarbenium (Trityl) Salts". Synthesis. 1972 (10): 544. doi:10.1055/s-1972-21914.
- ^ Fernandez-Galan, R.; Manzano, B; Otero, A; Lanfranchi, M; Pellinghelli, M. (1994). "19F and 31P NMR Evidence for Silver Hexafluorophosphate Hydrolysis in Solution". Inorg. Chem. 33 (10): 2309–2312. doi:10.1021/ic00088a039.