Tinyatoxin (TTX or TTN) is an analog of the neurotoxin resiniferatoxin. It occurs naturally in Euphorbia poissonii.[1]
| |
| Names | |
|---|---|
| Preferred IUPAC name
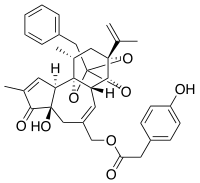
[(2S,3aR,3bS,6aR,9aR,9bR,10R,11aR)-2-Benzyl-6a-hydroxy-8,10-dimethyl-7-oxo-11a-(prop-1-en-2-yl)-3a,3b,6,6a,7,9a,11,11a-octahydro-2H,10H-2,9b-epoxyazuleno[5,4-e][1,3]benzodioxol-5-yl]methyl (4-hydroxyphenyl)acetate | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.165.094 |
| MeSH | tinyatoxin |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C36H38O8 | |
| Molar mass | 598.692 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
| Tinyatoxin | |
|---|---|
| Heat | Above peak |
| Scoville scale | 5,300,000,000 SHU |
It is a neurotoxin that acts via full agonism of the vanilloid receptors of sensory nerves.[2] Tinyatoxin has a potential for pharmaceutical uses similar to uses of capsaicin. Tinyatoxin is about one third as strong as resiniferatoxin but is still an ultrapotent analogue of capsaicin, with a heat intensity estimate of 300 to 350 times that of capsaicin.[citation needed]
References
edit- ^ Euphorbia poissonii in BoDD – Botanical Dermatology Database
- ^ Szallasi, A. & Blumberg, P.M. (1991). "Characterization of vanilloid receptors in the dorsal horn of pig spinal cord". Brain Res. 547 (2): 335–338. doi:10.1016/0006-8993(91)90982-2. PMID 1884211. S2CID 25052101.