Supercritical angle fluorescence microscopy (SAF) is a technique to detect and characterize fluorescent species (proteins, biomolecules, pharmaceuticals, etc.) and their behaviour close or even adsorbed or linked at surfaces. The method is able to observe molecules in a distance of less than 100 to 0 nanometer from the surface even in presence of high concentrations of fluorescent species around. Using an aspheric lens for excitation of a sample with laser light, fluorescence emitted by the specimen is collected above the critical angle of total internal reflection selectively and directed by a parabolic optics onto a detector. The method was invented in 1998 in the laboratories of Stefan Seeger at University of Regensburg/Germany and later at University of Zurich/Switzerland.

SAF microscopy principle
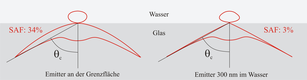
editThe principle how SAF Microscopy works is as follows: A fluorescent specimen does not emit fluorescence isotropically when it comes close to a surface, but approximately 70% of the fluorescence emitted is directed into the solid phase. Here, the main part enters the solid body above the critical angle.[1] When the emitter is located just 200 nm above the surface, fluorescent light entering the solid body above the critical angle is decreased dramatically. Hence, SAF Microscopy is ideally suited to discriminate between molecules and particles at or close to surfaces and all other specimen present in the bulk.[2][3]
Typical SAF-setup
editThe typical SAF setup consists of a laser line (typically 450-633 nm), which is reflected into the aspheric lens by a dichroic mirror. The lens focuses the laser beam in the sample, causing the particles to fluoresce. The fluorescent light then passes through a parabolic lens before reaching a detector, typically a photomultiplier tube or avalanche photodiode detector. It is also possible to arrange SAF elements as arrays, and image the output onto a CCD, allowing the detection of multiple analytes.[4]
Selected publications
edit- ^ J. Enderlein, T. Ruckstuhl, S. Seeger: Highly Efficient Optical Detection of Surface-Generated Fluorescence. Appl. Opt. 38 (4) 724-32 (1999)
- ^ T. Ruckstuhl, M. Rankl, S. Seeger: Highly sensitive biosensing using a Supercritical Angle Fluorescence (SAF) instrument, Biosensors&Bioelectronics 18 (9) 1193-1199 (2003)
- ^ T. Ruckstuhl, S. Seeger: Attoliter detection volumes by confocal total-internal-reflection fluorescence microscopy, Optic Letters 29, 569-571 (2004)
- ^ Hill, D; McDonnell B; Hearty S; Basabe-Desmonts L; Blue R; Trnavsky M; McAtamney C; O'Kennedy R; Maccraith B (11 May 2011). "Novel disposable biochip platform employing supercritical angle fluorescence for enhanced fluorescence collection". Biomedical Microdevices. 13 (4): 759–67. doi:10.1007/s10544-011-9546-2. PMID 21559870. S2CID 22221110.