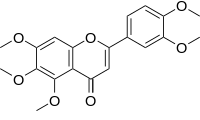
Sinensetin is a methylated flavone. It can be found in Orthosiphon stamineus[2] and in orange oil.[3]
| |

| |
| Names | |
|---|---|
| IUPAC name
3′,4′,5,6,7-Pentamethoxyflavone
| |
| Systematic IUPAC name
2-(3,4-Dimethoxyphenyl)-5,6,7-trimethoxy-4H-1-benzopyran-4-one | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.230.396 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C20H20O7 | |
| Molar mass | 372.36 g/mol |
| Hazards | |
| GHS labelling:[1] | |

| |
| Danger | |
| H301 | |
| P264, P270, P301+P310, P321, P330, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
References
edit- ^ GHS: Pubchem
- ^ Akowuah, G. A.; Ismail, Z.; Norhayati, I.; Sadikun, A. (2005). "The effects of different extraction solvents of varying polarities on polyphenols of Orthosiphon stamineus and evaluation of the free radical-scavenging activity". Food Chemistry. 93 (2): 311–317. doi:10.1016/j.foodchem.2004.09.028.
- ^ Steinke, Katrin; Jose, Elena; Sicker, Dieter; Siehl, Hans-Ullrich; Zeller, Klaus-Peter; Berger, Stefan (2013). "Sinensetin". Chemie in unserer Zeit (in German). 47 (3): 158–163. doi:10.1002/ciuz.201300627.