| Primary Carbon |
|---|
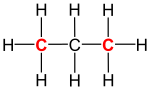
|
| Structural formula of propane (C3H8; primary carbons are highlighted red) |
In organic chemistry, a primary carbon is a carbon atom which is bound to only one other carbon atom.[1] It is thus at the end of a carbon chain. In case of an alkane, three hydrogen atoms are bound to a primary carbon (see propane in the figure on the right). A hydrogen atom could also be replaced by a hydroxy group (−OH), which would make the molecule a primary alcohol.[2]
| primary carbon | secondary carbon | tertiary carbon | quaternary carbon | |
| General structure (R = Organyl group) |

|

|

|

|
| Partial Structural formula |

|

|

|

|
References
edit- ^ Smith, Janice Gorzynski (2011). "Chapter 4 Alkanes". Organic chemistry (3rd ed.). New York, NY: McGraw-Hill. p. 116. ISBN 978-0-07-337562-5. Archived from the original (Book) on 2018-06-28. Retrieved 2018-06-26.
- ^ Hans Peter Latscha, Uli Kazmaier, Helmut Alfons Klein (2016), Organische Chemie: Chemie-Basiswissen II (in German) (7. Auflage ed.), Berlin: Springer Spektrum, p. 40, ISBN 978-3-662-46180-8
{{citation}}: CS1 maint: multiple names: authors list (link)