Praseodymium(IV) fluoride (also praseodymium tetrafluoride) is a binary inorganic compound, a highly oxidised metal salt of praseodymium and fluoride[1] with the chemical formula PrF4.
| |
| Names | |
|---|---|
| Other names
tetrafluoropraseodymium, praseodymium tetrafluoride
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| F4Pr | |
| Molar mass | 216.90127 g·mol−1 |
| Appearance | light-yellow crystals |
| Density | g/cm3 |
| reacts with water | |
| Hazards | |
| GHS labelling: | |

| |
| Warning | |
| Related compounds | |
Other cations
|
CeF4 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Synthesis
editPraseodymium(IV) fluoride can be prepared by the effect of krypton difluoride on praseodymium(IV) oxide:[2]
Praseodymium(IV) fluoride can also be made by the dissolution of sodium hexafluoropraseodymate(IV) in liquid hydrogen fluoride:[3]
Properties
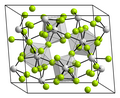
editPraseodymium(IV) fluoride forms light yellow crystals. The crystal structure is anticubic and isomorphic to that of uranium tetrafluoride UF4. It decomposes when heated:
Due to the high normal potential of the tetravalent praseodymium cations (Pr3+ / Pr4+: +3.2 V), praseodymium(IV) fluoride decomposes in water, releasing oxygen, O2.
See also
editReferences
edit- ^ Vent-Schmidt, Thomas; Riedel, Sebastian (November 6, 2015). "Investigation of Praseodymium Fluorides: A Combined Matrix-Isolation and Quantum-Chemical Study". Inorganic Chemistry. 54 (23): 11114–11120. doi:10.1021/acs.inorgchem.5b01175. PMID 26544761. Retrieved 18 June 2021.
- ^ Meyer, G.; Morss, Lester R. (1990-12-31). Synthesis of Lanthanide and Actinide Compounds. Springer Science & Business Media. p. 367. ISBN 978-0-7923-1018-1. Retrieved 18 June 2021.
- ^ Emeléus, H. J.; Sharpe, A. G. (1977-09-01). Advances in Inorganic Chemistry and Radiochemistry. Academic Press. p. 368. ISBN 978-0-08-057869-9. Retrieved 18 June 2021.