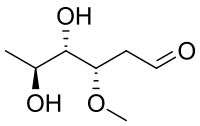
Oleandrose is a type of carbohydrate with the chemical formula C7H14O4. With a six-carbon chain, it is classified as a hexose. With two hydroxyl groups replaced with hydrogen atoms, it is a dideoxy sugar. The hydroxyl group at C3 is methylated.
| |
| Names | |
|---|---|
| IUPAC name
(3S,4S,5S)-4,5-Dihydroxy-3-methoxyhexanal
| |
| Other names
2,6-Dideoxy-3-O-methyl-L-arabinohexose
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C7H14O4 | |
| Molar mass | 162.185 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Occurrence
editOleandrdose is found in the leaves of Nerium oleander and may contribute to the toxicity of the plant.[1][2] Oleandrose is also a component of several naturally-occurring chemical compounds including the avermectins (emamectin, abamectins, ivermectin, and others), the macrolide antibiotic oleandomycin, and the cardiac glycoside oleandrin.
Laboratory syntheses of L-oleandrose[3] and DL-oleandrose[4] have been reported.
See also
edit- Sarmentose, a diastereomeric dideoxy sugar
References
edit- ^ Siddiqui, Bina Shaheen; Khatoon, Nasima; Begum, Sabira; Farooq, Ahsana Dar; Qamar, Kehkashan; Bhatti, Huma Aslam; Ali, Syed Kashif (2012). "Flavonoid and cardenolide glycosides and a pentacyclic triterpene from the leaves of Nerium oleander and evaluation of cytotoxicity". Phytochemistry. 77: 238–244. Bibcode:2012PChem..77..238S. doi:10.1016/j.phytochem.2012.01.001. PMID 22281382.
- ^ Bakir Çilesizoğlu, Neşe; Yalçin, Emine; Çavuşoğlu, Kültiğin; Sipahi Kuloğlu, Selin (2022). "Qualitative and quantitative phytochemical screening of Nerium oleander L. Extracts associated with toxicity profile". Scientific Reports. 12 (1): 21421. Bibcode:2022NatSR..1221421B. doi:10.1038/s41598-022-26087-0. PMC 9742154. PMID 36504046.
- ^ Bredenkamp, Martin W.; Holzapfel, Cedric W.; Toerien, Francois (1992). "Alternative Syntheses of L-(-)-Oleandrose from L-Rhamnose1Preparation of Glycals". Synthetic Communications. 22 (17): 2459–2477. doi:10.1080/00397919208021642.
- ^ Berti, G.; Catelani, G.; Colonna, F.; Monti, L. (1982). "A highly diastereoselective synthesis of dl-oleandrose". Tetrahedron. 38 (20): 3067–3072. doi:10.1016/0040-4020(82)80194-4.