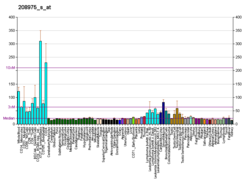
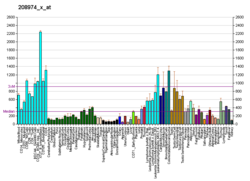
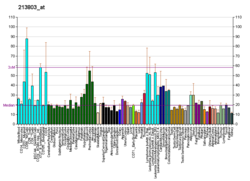
Importin subunit beta-1 is a protein that in humans is encoded by the KPNB1 gene.[5][6]
Function
editNucleocytoplasmic transport, a signal- and energy-dependent process, takes place through nuclear pore complexes embedded in the nuclear envelope. The import of proteins containing a classical nuclear localization signal (NLS) requires the NLS import receptor, a heterodimer of importin alpha and beta subunits. Each of these subunits are part of the karyopherin family of proteins. Importin alpha binds the NLS-containing cargo in the cytoplasm and importin beta docks the complex at the cytoplasmic side of the nuclear pore complex. In the presence of nucleoside triphosphates and the small GTP binding protein Ran, the complex moves into the nuclear pore complex and the importin subunits dissociate. Importin alpha enters the nucleoplasm with its passenger protein and importin beta remains at the pore. Interactions between importin beta and the FG repeats of nucleoporins are essential in translocation through the pore complex. The protein encoded by this gene is a member of the importin beta family.[7]
Interactions
editKPNB1 has been shown to interact with:
- KPNA3,[8][9]
- Karyopherin alpha 1,[8][10]
- Karyopherin alpha 2,[8][11][12]
- Mothers against decapentaplegic homolog 3,[13]
- NUP153[14][15][16]
- NUP50,[17]
- NUP98,[18][19]
- Nucleoporin 62,[10][14]
- P53,[20]
- Parathyroid hormone-related protein,[21][22]
- RANBP1,[23][24]
- RANBP2,[14][23][25]
- Ran (biology),[10][24][26] and
- SMN1.[27]
References
edit- ^ a b c GRCh38: Ensembl release 89: ENSG00000108424 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000001440 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Chi NC, Adam EJ, Adam SA (August 1995). "Sequence and characterization of cytoplasmic nuclear protein import factor p97". J. Cell Biol. 130 (2): 265–74. doi:10.1083/jcb.130.2.265. PMC 2199936. PMID 7615630.
- ^ Görlich D, Kostka S, Kraft R, Dingwall C, Laskey RA, Hartmann E, Prehn S (September 1995). "Two different subunits of importin cooperate to recognize nuclear localization signals and bind them to the nuclear envelope". Curr. Biol. 5 (4): 383–92. Bibcode:1995CBio....5..383G. doi:10.1016/S0960-9822(95)00079-0. hdl:11858/00-001M-0000-002D-1CBD-2. PMID 7627554. S2CID 6055941.
- ^ "Entrez Gene: KPNB1 karyopherin (importin) beta 1".
- ^ a b c Köhler M, Speck C, Christiansen M, Bischoff FR, Prehn S, Haller H, Görlich D, Hartmann E (November 1999). "Evidence for distinct substrate specificities of importin alpha family members in nuclear protein import". Mol. Cell. Biol. 19 (11): 7782–91. doi:10.1128/mcb.19.11.7782. PMC 84838. PMID 10523667.
- ^ Nachury MV, Ryder UW, Lamond AI, Weis K (January 1998). "Cloning and characterization of hSRP1 gamma, a tissue-specific nuclear transport factor". Proc. Natl. Acad. Sci. U.S.A. 95 (2): 582–7. Bibcode:1998PNAS...95..582N. doi:10.1073/pnas.95.2.582. PMC 18463. PMID 9435235.
- ^ a b c Percipalle P, Clarkson WD, Kent HM, Rhodes D, Stewart M (March 1997). "Molecular interactions between the importin alpha/beta heterodimer and proteins involved in vertebrate nuclear protein import". J. Mol. Biol. 266 (4): 722–32. doi:10.1006/jmbi.1996.0801. PMID 9102465.
- ^ Nagoshi E, Yoneda Y (April 2001). "Dimerization of sterol regulatory element-binding protein 2 via the helix-loop-helix-leucine zipper domain is a prerequisite for its nuclear localization mediated by importin beta". Mol. Cell. Biol. 21 (8): 2779–89. doi:10.1128/MCB.21.8.2779-2789.2001. PMC 86908. PMID 11283257.
- ^ Ullman KS, Powers MA, Forbes DJ (September 1997). "Nuclear export receptors: from importin to exportin". Cell. 90 (6): 967–70. doi:10.1016/s0092-8674(00)80361-x. PMID 9323123. S2CID 577884.
- ^ Xiao Z, Liu X, Lodish HF (August 2000). "Importin beta mediates nuclear translocation of Smad 3". J. Biol. Chem. 275 (31): 23425–8. doi:10.1074/jbc.C000345200. PMID 10846168.
- ^ a b c Ben-Efraim I, Gerace L (January 2001). "Gradient of increasing affinity of importin beta for nucleoporins along the pathway of nuclear import". J. Cell Biol. 152 (2): 411–7. doi:10.1083/jcb.152.2.411. PMC 2199621. PMID 11266456.
- ^ Nakielny S, Shaikh S, Burke B, Dreyfuss G (April 1999). "Nup153 is an M9-containing mobile nucleoporin with a novel Ran-binding domain". EMBO J. 18 (7): 1982–95. doi:10.1093/emboj/18.7.1982. PMC 1171283. PMID 10202161.
- ^ Kehlenbach RH, Gerace L (June 2000). "Phosphorylation of the nuclear transport machinery down-regulates nuclear protein import in vitro". J. Biol. Chem. 275 (23): 17848–56. doi:10.1074/jbc.M001455200. PMID 10749866.
- ^ Lindsay ME, Plafker K, Smith AE, Clurman BE, Macara IG (August 2002). "Npap60/Nup50 is a tri-stable switch that stimulates importin-alpha:beta-mediated nuclear protein import". Cell. 110 (3): 349–60. doi:10.1016/s0092-8674(02)00836-x. PMID 12176322. S2CID 15515037.
- ^ Moroianu J, Blobel G, Radu A (September 1997). "RanGTP-mediated nuclear export of karyopherin alpha involves its interaction with the nucleoporin Nup153". Proc. Natl. Acad. Sci. U.S.A. 94 (18): 9699–704. Bibcode:1997PNAS...94.9699M. doi:10.1073/pnas.94.18.9699. PMC 23253. PMID 9275187.
- ^ Bonifaci N, Moroianu J, Radu A, Blobel G (May 1997). "Karyopherin beta2 mediates nuclear import of a mRNA binding protein". Proc. Natl. Acad. Sci. U.S.A. 94 (10): 5055–60. Bibcode:1997PNAS...94.5055B. doi:10.1073/pnas.94.10.5055. PMC 24630. PMID 9144189.
- ^ Akakura S, Yoshida M, Yoneda Y, Horinouchi S (May 2001). "A role for Hsc70 in regulating nucleocytoplasmic transport of a temperature-sensitive p53 (p53Val-135)". J. Biol. Chem. 276 (18): 14649–57. doi:10.1074/jbc.M100200200. PMID 11297531.
- ^ Cingolani G, Bednenko J, Gillespie MT, Gerace L (December 2002). "Molecular basis for the recognition of a nonclassical nuclear localization signal by importin beta". Mol. Cell. 10 (6): 1345–53. doi:10.1016/s1097-2765(02)00727-x. PMID 12504010.
- ^ Lam MH, Hu W, Xiao CY, Gillespie MT, Jans DA (March 2001). "Molecular dissection of the importin beta1-recognized nuclear targeting signal of parathyroid hormone-related protein". Biochem. Biophys. Res. Commun. 282 (2): 629–34. doi:10.1006/bbrc.2001.4607. PMID 11401507.
- ^ a b Yaseen NR, Blobel G (September 1999). "GTP hydrolysis links initiation and termination of nuclear import on the nucleoporin nup358". J. Biol. Chem. 274 (37): 26493–502. doi:10.1074/jbc.274.37.26493. PMID 10473610.
- ^ a b Plafker K, Macara IG (May 2000). "Facilitated nucleocytoplasmic shuttling of the Ran binding protein RanBP1". Mol. Cell. Biol. 20 (10): 3510–21. doi:10.1128/mcb.20.10.3510-3521.2000. PMC 85643. PMID 10779340.
- ^ Delphin C, Guan T, Melchior F, Gerace L (December 1997). "RanGTP targets p97 to RanBP2, a filamentous protein localized at the cytoplasmic periphery of the nuclear pore complex". Mol. Biol. Cell. 8 (12): 2379–90. doi:10.1091/mbc.8.12.2379. PMC 25714. PMID 9398662.
- ^ Kutay U, Izaurralde E, Bischoff FR, Mattaj IW, Görlich D (March 1997). "Dominant-negative mutants of importin-beta block multiple pathways of import and export through the nuclear pore complex". EMBO J. 16 (6): 1153–63. doi:10.1093/emboj/16.6.1153. PMC 1169714. PMID 9135132.
- ^ Narayanan U, Ospina JK, Frey MR, Hebert MD, Matera AG (July 2002). "SMN, the spinal muscular atrophy protein, forms a pre-import snRNP complex with snurportin1 and importin beta". Hum. Mol. Genet. 11 (15): 1785–95. doi:10.1093/hmg/11.15.1785. PMC 1630493. PMID 12095920.
Further reading
edit- Bukrinsky MI, Haffar OK (1997). "HIV-1 nuclear import: in search of a leader". Front. Biosci. 2 (4): d578–87. doi:10.2741/A213. PMID 9366553.
- Bukrinsky MI, Haffar OK (1998). "HIV-1 nuclear import: matrix protein is back on center stage, this time together with Vpr". Mol. Med. 4 (3): 138–43. doi:10.1007/BF03401911. PMC 2230352. PMID 9562972.
- Christophe D, Christophe-Hobertus C, Pichon B (2000). "Nuclear targeting of proteins: how many different signals?". Cell. Signal. 12 (5): 337–41. doi:10.1016/S0898-6568(00)00077-2. PMID 10822175.
- Chook YM, Blobel G (2001). "Karyopherins and nuclear import". Curr. Opin. Struct. Biol. 11 (6): 703–15. doi:10.1016/S0959-440X(01)00264-0. PMID 11751052.
- Li L, Li HS, Pauza CD, Bukrinsky M, Zhao RY (2006). "Roles of HIV-1 auxiliary proteins in viral pathogenesis and host-pathogen interactions". Cell Res. 15 (11–12): 923–34. doi:10.1038/sj.cr.7290370. PMID 16354571.
- Bukrinsky MI, Sharova N, Dempsey MP, Stanwick TL, Bukrinskaya AG, Haggerty S, Stevenson M (1992). "Active nuclear import of human immunodeficiency virus type 1 preintegration complexes". Proc. Natl. Acad. Sci. U.S.A. 89 (14): 6580–4. Bibcode:1992PNAS...89.6580B. doi:10.1073/pnas.89.14.6580. PMC 49545. PMID 1631159.
- Bukrinsky MI, Haggerty S, Dempsey MP, Sharova N, Adzhubel A, Spitz L, Lewis P, Goldfarb D, Emerman M, Stevenson M (1993). "A nuclear localization signal within HIV-1 matrix protein that governs infection of non-dividing cells". Nature. 365 (6447): 666–9. Bibcode:1993Natur.365..666B. doi:10.1038/365666a0. PMC 9524217. PMID 8105392. S2CID 25678.
- Lounsbury KM, Richards SA, Perlungher RR, Macara IG (1996). "Ran binding domains promote the interaction of Ran with p97/beta-karyopherin, linking the docking and translocation steps of nuclear import". J. Biol. Chem. 271 (5): 2357–60. doi:10.1074/jbc.271.5.2357. PMID 8576188.
- Görlich D, Henklein P, Laskey RA, Hartmann E (1996). "A 41 amino acid motif in importin-alpha confers binding to importin-beta and hence transit into the nucleus". EMBO J. 15 (8): 1810–7. doi:10.1002/j.1460-2075.1996.tb00530.x. PMC 450097. PMID 8617226.
- Weis K, Ryder U, Lamond AI (1996). "The conserved amino-terminal domain of hSRP1 alpha is essential for nuclear protein import". EMBO J. 15 (8): 1818–25. doi:10.1002/j.1460-2075.1996.tb00531.x. PMC 450098. PMID 8617227.
- Moroianu J, Blobel G, Radu A (1996). "Nuclear protein import: Ran-GTP dissociates the karyopherin alphabeta heterodimer by displacing alpha from an overlapping binding site on beta". Proc. Natl. Acad. Sci. U.S.A. 93 (14): 7059–62. Bibcode:1996PNAS...93.7059M. doi:10.1073/pnas.93.14.7059. PMC 38935. PMID 8692944.
- Görlich D, Panté N, Kutay U, Aebi U, Bischoff FR (1996). "Identification of different roles for RanGDP and RanGTP in nuclear protein import". EMBO J. 15 (20): 5584–94. doi:10.1002/j.1460-2075.1996.tb00943.x. PMC 452303. PMID 8896452.
- Chi NC, Adam EJ, Adam SA (1997). "Different binding domains for Ran-GTP and Ran-GDP/RanBP1 on nuclear import factor p97". J. Biol. Chem. 272 (10): 6818–22. doi:10.1074/jbc.272.10.6818. PMID 9045717.
- Percipalle P, Clarkson WD, Kent HM, Rhodes D, Stewart M (1997). "Molecular interactions between the importin alpha/beta heterodimer and proteins involved in vertebrate nuclear protein import". J. Mol. Biol. 266 (4): 722–32. doi:10.1006/jmbi.1996.0801. PMID 9102465.
- Kutay U, Izaurralde E, Bischoff FR, Mattaj IW, Görlich D (1997). "Dominant-negative mutants of importin-beta block multiple pathways of import and export through the nuclear pore complex". EMBO J. 16 (6): 1153–63. doi:10.1093/emboj/16.6.1153. PMC 1169714. PMID 9135132.
- Bonifaci N, Moroianu J, Radu A, Blobel G (1997). "Karyopherin beta2 mediates nuclear import of a mRNA binding protein". Proc. Natl. Acad. Sci. U.S.A. 94 (10): 5055–60. Bibcode:1997PNAS...94.5055B. doi:10.1073/pnas.94.10.5055. PMC 24630. PMID 9144189.
- Görlich D, Dabrowski M, Bischoff FR, Kutay U, Bork P, Hartmann E, Prehn S, Izaurralde E (1997). "A novel class of RanGTP binding proteins". J. Cell Biol. 138 (1): 65–80. doi:10.1083/jcb.138.1.65. PMC 2139951. PMID 9214382.
- Tiganis T, Flint AJ, Adam SA, Tonks NK (1997). "Association of the T-cell protein tyrosine phosphatase with nuclear import factor p97". J. Biol. Chem. 272 (34): 21548–57. doi:10.1074/jbc.272.34.21548. PMID 9261175.