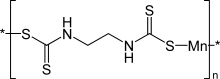
Maneb (manganese ethylene-bis-dithiocarbamate) is a fungicide and a polymeric complex of manganese with the ethylene bis (dithiocarbamate) anionic ligand.[1]
| |
| Names | |
|---|---|
| IUPAC name
[[2-[(Dithiocarboxy)amino]ethyl]carbamodithioato]](2-)-kS,kS']manganese
| |
| Other names
Manganese ethylene-1,2-bisdithiocarbamate, polymer
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.032.400 |
| MeSH | Maneb |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| (C4H6MnN2S4)n | |
| Appearance | Yellow to brown colored crystalline solid |
| Density | 1.92 g/cm3 |
| Melting point | 192 to 204 °C (378 to 399 °F; 465 to 477 K) (decomposes) |
| 160 mg/L | |
| Hazards | |
| GHS labelling: | |
  
| |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Health effects
editExposure to Maneb can occur when breathed in, it can irritate the eyes, nose, and throat as well as headache, fatigue, nervousness, dizziness, seizures and even unconsciousness. Prolonged or long-term exposure may interfere with the function of the thyroid. Exposure to Maneb along with Paraquat is also linked to Parkinson's disease, although the statement is still challenged.[2][3]
Production
editManganese(II) ethylenebis(dithiocarbamate) of low ethylenethiourea (ETU) content is prepared by mixing disodium ethylenebis (dithiocarbamate) with formaldehyde in aqueous medium then mixing a water-soluble manganese(II) salt to precipitate the maneb. The product can be further formulated with a metal salt and also with paraformaldehyde. (See External links for the patent citation)
Applications
editManeb, is a broad spectrum fungicide that is extensively applied against a wide range of fungal pathogens affecting ornamental plants, food and feed crops. It can also be used to create a toxin-based animal model of Parkinson's disease, usually in primates.[4][5]
Environmental effects
editThis section is empty. You can help by adding to it. (August 2024) |
Regulation
editIt was included in a pesticide ban proposed by the Swedish Chemicals Agency [6] and approved by the European Parliament on January 13, 2009.[7]
See also
edit- Metam sodium - A related dithiocarbamate salt which is also used as a fungicide.
- Zineb - ethylene bis(dithiocarbamate) with zinc instead of manganese.
- Mancozeb - A common fungicide containing Zineb and Maneb.
References
edit- ^ Reidies AH (June 2000). "Manganese compounds.". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a16_123. ISBN 978-3-527-30385-4.
- ^ "MANEB" (PDF). Hazardous Substance Fact Sheet. New Jersey Department of Health and Senior Services.
- ^ Costello S, Cockburn M, Bronstein J, Zhang X, Ritz B (April 2009). "Parkinson's disease and residential exposure to maneb and paraquat from agricultural applications in the central valley of California". American Journal of Epidemiology. 169 (8): 919–926. doi:10.1093/aje/kwp006. PMC 2727231. PMID 19270050.
- ^ Garcinuño RM, Fernández-Hernando P, Cámara C (July 2004). "Simultaneous determination of maneb and its main metabolites in tomatoes by liquid chromatography using diode array ultraviolet absorbance detection". Journal of Chromatography. A. 1043 (2): 225–229. doi:10.1016/j.chroma.2004.05.059. PMID 15330096.
- ^ Cicchetti F, Drouin-Ouellet J, Gross RE (September 2009). "Environmental toxins and Parkinson's disease: what have we learned from pesticide-induced animal models?". Trends in Pharmacological Sciences. 30 (9): 475–483. doi:10.1016/j.tips.2009.06.005. PMID 19729209.
- ^ "Interpretation of criteria for approval of active substances in the proposed EU plant protection regulation". Swedish Chemicals Agency (KemI). 2008-09-23. Archived from the original on 2009-01-01. Retrieved 2009-01-14.
- ^ "MEPs approve pesticides legislation". 2009-01-13. Archived from the original on 2009-01-25. Retrieved 2009-01-14.
External links
edit- Maneb in the Pesticide Properties DataBase (PPDB)
- US 4217293A, Adams, John B, "Stabilized maneb and preparation thereof", published 1980-08-12, issued 1980-08-12 and EIDP Inc