Isononyl alcohol (INA) is a nine carbon primary alcohol. It is used in small amounts as fragrance in soap, hair spray, face creams, and shampoo. INA, along with 3,5,5-Trimethyl-1-hexanol, makes up the mixture sometimes referred to as isononanol.[2]
| |
| Names | |
|---|---|
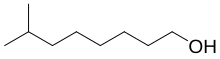
| IUPAC name
7-methyloctan-1-ol
| |
| Identifiers | |
3D model (JSmol)
|
|
| 4-01-00-01806 | |
| ChEBI | |
| ChemSpider | |
| EC Number |
|
| MeSH | alcohol isononyl alcohol |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C9H20O | |
| Molar mass | 144.258 g·mol−1 |
| Appearance | Clear liquid |
| Density | 0.83 g/cm3 |
| Boiling point | 215 °C |
| Slightly soluble | |
| Vapor pressure | 0.0198 mm Hg |
Henry's law
constant (kH) |
0.0000412 atm m3/mol |
| Hazards | |
| GHS labelling:[1] | |
 
| |
| Warning | |
| H315, H318, H319, H412 | |
| P264, P264+P265, P273, P280, P302+P352, P305+P351+P338, P305+P354+P338, P317, P321, P332+P317, P337+P317, P362+P364, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | 98 °C |
| Related compounds | |
Related compounds
|
3,5,5-Trimethyl-1-hexanol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Nonyl alcohols, including isononyl alcohol, are typically produced by hydroformylation of octenes. Isomeric octenes are produced by dimerization of butenes. These alcohol mixtures are used as solvents in paints and as precursors to plasticizers.[3]
References
edit- ^ "Isononyl alcohol". pubchem.ncbi.nlm.nih.gov.
- ^ McGinty, D.; Scognamiglio, J.; Letizia, C. S.; Api, A. M. (2010-07-01). "Fragrance material review on isononyl alcohol". Food and Chemical Toxicology. A Safety Assessment of Saturated Branched Chain Alcohols when used as Fragrance Ingredients. 48: S79–S81. doi:10.1016/j.fct.2010.05.034. ISSN 0278-6915. PMID 20659642.
- ^ Falbe, Jürgen; Bahrmann, Helmut; Lipps, Wolfgang; Mayer, Dieter; Frey, Guido D. (2013). "Alcohols, Aliphatic". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a01_279.pub2. ISBN 978-3-527-30385-4.
