Homovanillyl alcohol is a metabolite of hydroxytyrosol, which in turn is a metabolite of the neurotransmitter dopamine.
| |
| Names | |
|---|---|
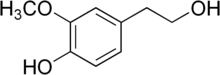
| Preferred IUPAC name
4-(2-Hydroxyethyl)-2-methoxyphenol | |
| Other names
Homovanillic alcohol; MOPET; 3-Methoxy-4-hydroxyphenylethanol; 3-Methoxy-4-hydroxyphenethyl alcohol; 4-Hydroxy-3-methoxyphenethanol, 4-Hydroxy-3-methoxyphenethyl alcohol
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.017.433 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C9H12O3 | |
| Molar mass | 168.19 g/mol |
| Melting point | 40 to 42 °C (104 to 108 °F; 313 to 315 K) |
| Hazards | |
| GHS labelling:[2] | |

| |
| Warning | |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
| Flash point | 113 °C (235 °F; 386 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
See also
editReferences
edit- ^ Homovanillyl alcohol at Sigma-Aldrich
- ^ "Homovanillyl alcohol". pubchem.ncbi.nlm.nih.gov. Retrieved 13 December 2021.